Поддержка лютеиновой фазы (ЛФ) – неотъемлемый этап программ вспомогательных репродуктивных технологий (ВРТ), оказывающий существенное влияние на их эффективность. Стимуляция суперовуляции в программе экстракорпорального оплодотворения (ЭКО) сопровождается супрафизиологическими уровнями стероидных гормонов, секретируемых большим количеством желтых тел в начале ЛФ, которые подавляют секрецию лютеинизирующего гормона (ЛГ) по механизму отрицательной обратной связи, что приводит к недостаточности ЛФ [1, 2]. Недостаточность ЛФ оказывает отрицательное влияние на раннее функционирование желтого тела, что может быть причиной неудач имплантации в программе ЭКО. Поддержка ЛФ является обязательным этапом в программе ЭКО в протоколах как с агонистами гонадотропин-рилизинг гормона (аГнРГ), так и с антагонистами гонадотропин-рилизинг гормона (антГнРГ) и имеет решающее значение, особенно в период времени между снижением экзогенного хорионического гонадотропина (ХГч), назначаемого для финального созревания ооцитов, и повышением эндогенного ХГч на этапе имплантации эмбриона. Для восполнения недостаточности ЛФ используются различные режимы ее поддержки, включающие препараты прогестерона, эстрадиола (E2), ХГч и их комбинации, которые часто назначаются эмпирически, поскольку не существует идеального режима ведения ЛФ, что вызывает необходимость продолжения исследований для разработки оптимального режима поддержки ЛФ в программе ЭКО.
Относительно новым и малоизученным является комбинированный метод поддержки ЛФ в программах ВРТ с использованием аГнРГ в сочетании с микронизированным прогестероном. Несмотря на то, что положительное влияние аГнРГ в ЛФ на исходы программ ВРТ подтверждено рядом клинических исследований [3–6], не существует однозначного мнения об эффективности данного метода поддержки ЛФ, не изучен таргетный механизм действия аГнРГ в ЛФ в программе ЭКО. Не определены группы пациенток, у которых использование данного режима поддержки ЛФ наиболее целесообразно.
Целью настоящего рандомизированного контролируемого клинического исследования (РКИ) стала сравнительная оценка влияния комбинированного режима поддержки ЛФ с добавлением аГнРГ к микронизированному прогестерону и стандартного режима поддержки ЛФ микронизированным прогестероном на эффективность программы ЭКО.
Материал и методы исследования
Исследование проведено у 207 пациенток в возрасте до 38 лет с трубно-перитонеальным, мужским или сочетанными факторами бесплодия. Критериями включения в исследование были: уровень фолликулостимулирующего гормона (ФСГ) <12 МЕ/л, регулярный менструальный цикл 21–35 дней, не более 2 безуспешных попыток ЭКО в анамнезе. Критерии невключения: наружный и внутренний эндометриоз III–IV степени распространения, интерстициальная или субсерозная миома матки размером более 4 см, субмукозная миома матки, пороки развития внутренних половых органов, патозооспермия III–IV степени, развитие синдрома гиперстимуляции яичников средней или тяжелой степени в данном цикле ЭКО. Информированное согласие на участие в исследовании было получено у всех пациенток.
На этапе подготовки к программе ЭКО у 41 пациентки с бесплодием, имеющих неудачные попытки ЭКО в анамнезе, и у 14 фертильных женщин в период «окна имплантации» естественного цикла (пик ЛГ+7) с помощью иммуногистохимического (ИГХ) исследования оценивали экспрессию ГнРГ и рецептора ГнРГ в пайпель-биоптатах эндометрия.
Стимуляцию суперовуляции проводили в фиксированном протоколе с антГнРГ препаратами рекомбинантного фолликулостимулирующего гормона со 2–3-го дня менструального цикла. Подбор стартовой дозы гонадотропинов осуществляли с учетом параметров овариального резерва пациенток (уровень ФСГ, антимюллеров гормон (АМГ), число антральных фолликулов), возраста женщины и овариального ответа на предыдущие стимуляции суперовуляции. Дозу гонадотропинов корректировали в соответствии с ответом яичников на стимуляцию. В качестве триггера овуляции для финального созревания ооцитов назначали препараты ХГЧ 10 000 МЕ при визуализации трех и более фолликулов ≥17 мм в диаметре и толщине эндометрия 8–10 мм. Трансвагинальную пункцию (ТВП) яичников проводили через 35–36 часов после введения триггера овуляции. Преинкубация, оплодотворение ооцитов, а также культивирование эмбрионов осуществляли в средах для культивирования компании «ORIGIO» (Дания). Оценка качества полученных ооцитов по степени зрелости и эмбрионов осуществлялась на основании общепринятых критериев. Перенос 1–2 эмбрионов проводили на 3–5-е сутки культивирования. В день ТВП пациентки методом простой рандомизации были разделены на две группы в зависимости от режима поддержки ЛФ: пациентки 1-й группы (n=92) получали натуральный микронизированный прогестерон – 600 мг/день вагинально с 1-х суток после ТВП и однократную дозу трипторелина – 0,1 мг подкожно на 6-е сутки после ТВП; пациентки 2-й группы (n=115) для поддержки ЛФ получали только микронизированный прогестерон – 600 мг/день вагинально с 1-х суток после ТВП. У всех пациенток исследовали концентрации ЛГ, прогестерона, Е2 и βХГЧ в сыворотке крови на 5-й, 7-й и 15-й дни после ТВП. В ходе исследования оценивали стартовую и суммарную дозы гонадотропинов, продолжительность гонадотропной стимуляции; число полученных и зрелых ооцитов; частоту оплодотворения, качество эмбрионов; количество и качество перенесенных эмбрионов. Эффективность лечения оценивали по показателям частоты наступления беременности на перенос эмбрионов. Биохимическая беременность была диагностирована на 15-й день после ТВП при уровне βХГЧ в сыворотке крови >20 МЕ/л. Имплантацию подтверждали при визуализации плодного яйца в полости матки по данным ультразвукового исследования. Частота имплантации оценивалась как отношение числа плодных яиц к числу перенесенных эмбрионов. Клиническую беременность подтверждали при визуализации в полости матки эмбрионов с сердцебиением через 4–6 недель после переноса. Прогрессирующей считалась беременность, продолжающаяся после 12 недель после переноса эмбрионов. Статистическая обработка данных выполнена на персональном компьютере с использованием программы IPM SРSS Statistics, версия 21.
Результаты исследования
Анализ клинико-лабораторных характеристик пациенток, включенных в исследование, не выявил статистически значимых различий между группами по возрасту (медиана 32 года (интерквартильный интервал 29–35 лет), р=0,749). Базальные уровни гормонов на 2–3-й день менструального цикла были сопоставимы в двух группах: ФСГ (медиана 7,0 и 7,1 МЕ/л, р=0,789), ЛГ (медиана 4,2 и 4,0 МЕ/л, р=0,309) и Е2 (медиана 104 и 89 пмоль/л, р=0,113). Концентрации АМГ были несколько выше в 1-й группе, однако различия не были статистически значимы (медиана 2,6 и 2,2 нг/мл, р=0,518). Бесплодие было обусловлено трубно-перитонеальным фактором у 35,9% пациенток 1-й группы и у 35,7% женщин 2-й группы (р=0,974); мужским – у 44,6 и у 41,7% (р=0,684); сочетание факторов наблюдалось у 19,6 и 21,7% женщин (р=0,576). Первичное и вторичное бесплодие в обеих группах встречалось с одинаковой частотой (р=0,486), длительность бесплодия в 1-й группе составила 5,5±0,4 года, во 2-й группе – 5,7±0,3 года (р=0,446).
Параметры стимулированного цикла и эмбриогенеза в обеих группах представлены в табл. 1.
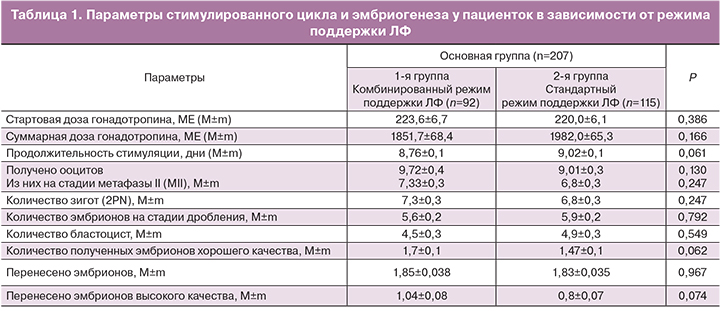
Сравнительный анализ протоколов стимуляции показал отсутствие значимых различий между группами в стартовых дозах гонадотропинов (223,6±6,7МЕ и 220,0±6,1 МЕ, р=0,386), суммарных дозах гонадотропинов (1851,7±68,4 МЕ и 1982,0±65,3 МЕ, р=0,166) и продолжительности овариальной стимуляции (8,76±0,1 и 9,02±0,1 дня; р=0,061).
Эмбриологические параметры в двух группах сравнения были сопоставимы по количеству полученных ооцитов (9,72±0,4 и 9,01±0,3; р=0,130), зрелых ооцитов (МII) (7,33±0,3 и 6,8±0,3; р=0,247) и зигот (2 PN) (7,3±0,3 и 6,8±0,3; р=0,814). Не наблюдалось межгрупповых различий в частоте дробления (5,6±0,2 и 5,9±0,2; р=0,792), развития эмбрионов до стадии бластоцисты (4,5±0,3 и 4,9±0,3; р=0,549), количестве полученных эмбрионов хорошего качества (1,7±0,1 и 1,47±0,1; р=0,062), перенесенных эмбрионов (1,85±0,03 и 1,83±0,03; р=0,967) и перенесенных эмбрионов хорошего качества (1,04±0,08 и 0,8±0,07; р=0,047).
Таким образом, пациентки обеих групп были сопоставимы по исходным клинико-лабораторным характеристикам, параметрам стимулированного цикла, оогенеза и раннего эмбриогенеза.
Результаты анализа эффективности программы ЭКО в зависимости от режима поддержки ЛФ (табл. 2) показали, что у пациенток с комбинированным режимом поддержки ЛФ по сравнению с пациентками со стандартным режимом, выявлена более высокая частота биохимической беременности (51,1 и 34,8%, р=0,02), имплантации (30,6 и 16,6%, р<0,05), клинической беременности (40,2 и 26,9%, р=0,04), прогрессирующей беременности (36,9 и 21,7%, р=0,01) и родов живым плодом (31,5 и 19,1%, р=0,04), что свидетельствует о позитивном влиянии комбинированного режима поддержки ЛФ на исходы программы ЭКО. Частота ранних репродуктивных потерь была сопоставима в обеих группах (8,7 и 7,8%, р=0,82). Вместе с тем следует отметить, что в группе женщин с комбинированным режимом поддержки ЛФ, по сравнению с группой пациенток со стандартным режимом поддержки ЛФ, отмечена более высокая частота многоплодной беременности (14,1 и 4,3%, р=0,005) и, как следствие, более высокая частота преждевременных родов (7,6 и 1,7%, р=0,03).

Таким образом, несмотря на отсутствие различий в клинико-анамнестических данных, параметрах стимулированного цикла и эмбриологических показателях в двух группах, выявлена более высокая эффективность программы ЭКО у пациенток, использовавших комбинированный режим поддержки ЛФ.
Анализ концентраций гормонов в сыворотке крови в зависимости от режима поддержки ЛФ показал, что на 7-й день после ТВП у пациенток, получавших комбинированный режим поддержки ЛФ, по сравнению с группой женщин со стандартным режимом поддержки ЛФ были выявлены более высокие уровни ЛГ (медиана 13,9 и 0,1 МЕ/л, р=0,0001), Е2 (медиана 5126 и 3501 пмоль/л, р=0,002) и прогестерона (медиана 335 и 170 нмоль/л, р=0,0001). Существенное повышение уровней ЛГ, прогестерона и Е2 в сыворотке крови пациенток с комбинированным режимом поддержки ЛФ, по сравнению с аналогичными показателями у женщин со стандартным режимом поддержки ЛФ свидетельствует о позитивном влиянии аГнРГ на функцию желтого тела.
Для оценки влияния аГнРГ, используемого в ЛФ на эмбрион, мы провели сравнительную оценку концентраций βХГч у пациенток с наступившей беременностью в зависимости от режима поддержки ЛФ. При комбинированном режиме поддержки ЛФ, по сравнению со стандартным режимом было выявлено достоверное повышение уровня βХГч на 15-й день после оплодотворения, как при одноплодных (медиана 220 и 112 Ме/л), так и при двухплодных беременностях (медиана 453 и 244 Ме/л), р<0,05, что косвенно подтверждает возможность влияния аГнРГ на эмбрион.
Результаты ИГХ исследования биоптатов эндометрия, полученных в период «окна имплантации» у пациенток с бесплодием и неудачными попытками ЭКО в анамнезе перед проведением данной программы ЭКО, показали, что в среднем экспрессия ГнРГ у пациенток с бесплодием была значимо ниже, чем у фертильных женщин в поверхностном эпителии (2,9 и 5,1 балла), в эпителии желез (1,9 и 3,7 балла) и в строме эндометрия (1,3 и 2,6 балла) (р<0,05). Экспрессия рецептора ГнРГ у пациенток с бесплодием также была существенно ниже, чем у фертильных женщин в поверхностном эпителии (0,9 и 3,8 балла), в железах (0,8 и 3,3 балла) в строме эндометрия (0,7 и 3,2 балла), р<0,05.
Результаты сравнительного анализа экспрессии ГнРГ и рецептора ГнРГ в эндометрии в зависимости от режима поддержки ЛФ показали сопоставимое снижение исследуемых маркеров у пациенток с бесплодием в группе комбинированного и стандартного режимов поддержки ЛФ (р>0,05).
Однако частота клинической беременности у пациенток со снижением экспрессии ГнРГ и рецептора ГнРГ в эндометрии была существенно выше в группе женщин с комбинированным режимом, по сравнению со стандартным режимом поддержки ЛФ (45 и 15%, р=0,03), что может свидетельствовать о позитивном влиянии аГнРГ на эндометрий у пациенток с бесплодием и неудачными попытками ЭКО в анамнезе.
Обсуждение
Данное рандомизированное контролируемое клиническое исследование было проведено с целью сравнительной оценки влияния комбинированного режима поддержки ЛФ (аГнРГ+микронизированный прогестерон) и стандартного режима (микронизированный прогестерон) на эффективность программы ЭКО в протоколе с антГнРГ. Проведенный анализ показал более высокую эффективность программы ЭКО при комбинированном режиме поддержки ЛФ по сравнению со стандартным режимом, о чем свидетельствовала более высокая частота биохимической беременности, имплантации, клинической беременности и прогрессирующей беременности у пациенток, использовавших аГнРГ для поддержки ЛФ, что согласуется с результатами исследования Tesařík (2006) [7]. Частота самопроизвольного прерывания беременности была сопоставима в обеих группах, что свидетельствует об отсутствии влияния аГнРГ на частоту ранних репродуктивных потерь и подтверждено результатами исследований Fuji и соавт. (2001), H. Qublan и соавт. (2008), Inamdar и соавт. (2011) [8–10]. В нашем исследовании отмечено значимое повышение частоты многоплодной беременности в группе с аГнРГ по сравнению с группой пациенток, использовавших для поддержки ЛФ микронизированный Р. Влияние аГнРГ на повышение частоты наступления многоплодной беременности было также отмечено в исследованиях Tesarik и соавт. (2006), Fuji и соавт. (2001), Isik и соавт. (2009) [3, 8, 11]. Обращала на себя внимание более высокая частота преждевременных родов в группе пациенток, использовавших аГнРГ для поддержки ЛФ (7,6 и 1,7%, р=0,03), что, по-видимому, обусловлено более высокой частотой многоплодной беременности и свидетельствует о целесообразности проведения селективного переноса одного эмбриона при использовании аГнРГ для поддержки ЛФ в программе ЭКО.
Вопросам оценки эффективности использования аГнРГ для поддержки ЛФ посвящен ряд систематических обзоров и мета-анализов. Oliveira и соавт. (2010) проанализировали результаты 5 РКИ, в которых для поддержки ЛФ использовали однократную дозу аГнРГ на 5-й или 6-й день после оплодотворения методом интрацитоплазматической инъекции сперматозоида в ооцит (ИКСИ). Отмечено значительное повышение частоты имплантации (р<0,0001), частоты клинической беременности на перенос эмбриона (р=0,006) и частоты прогрессирующей беременности (р=0,02) в группе пациенток, использовавших аГнРГ в ЛФ, по сравнению с не использовавшими аГнРГ. В длинных протоколах частота клинической беременности (р=0,06) и частота прогрессирующей беременностей (р=0,23) была сопоставима между группами, но частота имплантации была значимо выше в группе с аГнРГ(р=0,02). В протоколах с антГнРГ добавление аГнРГ в ЛФ приводило к повышению частоты имплантации (р=0,0002), частоты клинической беременности (р=0,04) и частоты прогрессирующей беременности (р=0,04). Авторы этого мета-анализа пришли к выводу, что назначение однократной дозы аГнРГ в ЛФ повышает частоту имплантации во всех протоколах и частоту клинической беременности и прогрессирующей беременности в протоколах с антГнРГ. Однако из-за немногочисленности и гетерогенности исследований они рекомендовали продолжить изучение этого режима поддержки ЛФ [3]. В систематическом обзоре и мета-анализе Kyrou и соавт. (2011) были проанализированы 6 РКИ (2012 женщин). Частота родов живым плодом была на 16% выше у пациенток, получавших аГнРГ для поддержки ЛФ (разность рисков: +16%, 95% ДИ: +10 – +22%), что позволило авторам сделать вывод, что добавление аГнРГ к микронизированному Р в ЛФ в программе ЭКО/ИКСИ значительно увеличивает вероятность родов живым плодом [5].
В обзоре Кохрейновского сообщества (2015) в семи РКИ оценивали эффективность использования аГнРГ для поддержки ЛФ. Частота родов живым плодом и частота прогрессирующей беременности были выше в группе женщин, использовавших микронизированный прогестерон + аГнРГ (OР 0,67, 95% ДИ 0,56–0,81; I(2)=69%). Качество доказательности было низким из-за существенной гетерогенности исследований, однако позитивный эффект от использования аГнРГ в ЛФ наблюдался во всех из них [6]. В систематическом обзоре и мета-анализе, проведенном Martins и соавт. (2016), проанализированы 8 РКИ (2776 женщин), результаты которых показали позитивное влияние аГнРГ на частоту живорождений и прогрессирующей беременности (ОР 1,26, 95% ДИ 1,04–1,53; I2=58%), при этом ни в одном из включенных исследований не отмечались неблагоприятные перинатальные исходы или врожденные пороки развития плода у женщин, использовавших аГнРГ для поддержки ЛФ [12].
Несмотря на позитивный эффект режима поддержки ЛФ с добавлением однократной дозы аГнРГ на исходы ВРТ, отмеченный в нашем исследовании и работах зарубежных коллег, до настоящего времени не ясен механизм действия аГнРГ в ЛФ в программе ЭКО. Обсуждаются три гипотезы о влиянии аГнРГ на желтое тело, эндометрий и эмбрион.
Вопрос о механизме действия аГнРГ на желтое тело обсуждался в нескольких работах. В исследовании Pirard и соавт. (2005) было показано, что низкие дозы аГнРГ (100 мкг бусерелина) способны оказывать стимулирующее действие на желтое тело [13]. В работе Tesarik и соавт. (2006) было выявлено повышение концентраций прогестерона и Е2, но не отмечено повышения уровня ЛГ на следующий день после введения аГнРГ, что не указывает на роль гипофиза в механизме действия аГнРГ. Однако необходимо учитывать тот факт, что для получения этого эффекта достаточно даже короткого пика ЛГ, который мог не быть зарегистрирован с учетом дизайна исследования [3].
Напротив, другими исследователями показано, что аГнРГ повышает продукцию ЛГ, предотвращая преждевременный лютеолиз, что приводит к повышению частоты наступления беременности [5].
В нашем исследовании выявлено значимое увеличение концентраций ЛГ, прогестерона и Е2 в сыворотке крови у пациенток с комбинированным режимом поддержки ЛФ, что отражает позитивное влияние аГнРГ на функцию желтого тела через центральные механизмы регуляции.
Возможность прямого влияния аГнРГ на эмбрион обсуждалась в ряде работ. В исследовании Klemmt (2009) показана роль аГнРГ как потенциального активатора развития эмбрионов и имплантации [14]. Установлено, что аГнРГ может воздействовать непосредственно на преимплантационный эмбрион, так как рецептор ГнРГ активно экспрессируется на эмбрионе человека от стадии морулы до стадии бластоцисты [15]. В экспериментальных исследованиях было показано ускорение развития свиных и мышиных эмбрионов при инкубации с аГнРГ и замедление при их инкубации с антГнРГ [16, 17].
В клиническом исследовании Tesarik и соавт., (2004) установлено, что использование аГнРГ для поддержки ЛФ повышает частоту наступления беременности в циклах донации ооцитов у реципиентов с подавленной овуляцией и отсутствием желтого тела, что предполагает прямое влияние аГнРГ на эмбрион [3]. Возможное прямое воздействие аГнРГ на эмбрион подтверждается и в другом исследовании Tesarik и соавт. (2006), в котором пациентки с наступившей беременностью в группе аГнРГ имели более высокие уровни βХГч на 15-й день после оплодотворения [7]. В нашей работе у пациенток, использовавших аГнРГ для поддержки ЛФ, было отмечено существенное повышение частоты имплантации и увеличение уровня βХГч на 15-й день после оплодотворения (р=0,0001), что свидетельствует о влиянии аГнРГ на эмбрион.
Однако нельзя исключить и прямое влияние аГнРГ на эндометрий. В работе H. Qublan (2008) было выявлено благоприятное влияние аГнРГ на эндометрий [9], которое связывают с экспрессией рецептора ГнРГ в течение менструального цикла в эпителиальных и стромальных клетках эндометрия, при этом максимальная экспрессия рецептора ГнРГ отмечается в ЛФ [18, 19]. Локальная экспрессия ГнРГ и его рецептора трофобластом играет важную роль в диалоге между бластоцистой и эндометрием на стадии ранней имплантации, что осуществляется путем модуляции баланса между матриксными металлопротеиназами и их ингибиторами [17]. Благодаря специфическому ингибированию тканевых ингибиторов металлопротеиназ повышается инвазивная способность трофобласта, что способствует более успешной имплантации бластоцисты. Тем не менее, in vitro не удалось выявить существенного влияния аГнРГ на степень децидуализации эндометриальных стромальных клеток [14] и на экспрессию генов эпителиальных клеток эндометрия человека [20]. В нашем исследовании впервые проведена оценка экспрессии ГнРГ и рецептора ГнРГ в биоптатах эндометрия в «окно имплантации» у женщин с бесплодием, которая выявила существенное снижение экспрессии этих маркеров в поверхностном эпителии, эпителии желез и строме эндометрия по сравнению с фертильными женщинами. Результаты сравнительного анализа экспрессии ГнРГ и его рецептора в эндометрии в зависимости от режима поддержки ЛФ показали сопоставимое снижение исследуемых маркеров у пациенток с бесплодием как при комбинированном, так и при стандартном режимах поддержки ЛФ (р>0,05). Однако последующий анализ показал, что частота клинической беременности была существенно выше в группе женщин с комбинированным режимом по сравнению со стандартным режимом поддержки ЛФ (45 и 15%, р=0,03), что может свидетельствовать о влиянии аГнРГ на эндометрий.
Несмотря на позитивные результаты влияния аГнРГ, используемого для поддержки ЛФ, на исходы программ ВРТ, до сих пор не решен вопрос об оптимальной дозе, пути введения и продолжительности использования аГнРГ в ЛФ в программе ЭКО. Однократное введение аГнРГ для поддержки ЛФ на 6-й день после оплодотворения было отмечено в большинстве работ [7, 11, 21–23]; в исследовании Inamdar (2012) аГнРГ назначали на 6-й, 7-й и 8-й дни после трансвагинальной пункции (ТВП) [10]; в работе Qublan и соавт. (2008) аГнРГ вводили в день оплодотворения, затем в день переноса эмбриона и в последующие три дня [9], а в исследованиях Isikoglu и соавт., (2007) и Fujii и соавт. (2009) аГнРГ назначали ежедневно до 12–14-го дня после ТВП [8, 24]. В большинстве проведенных исследований применяли 0,1 мг трипторелина [7, 11, 21–23, 25], в исследовании Inamdar (2011) – 1 мг лейпролида ацетата [10], а в работе Isik и соавт. (2009) – 0,5 мг лейпролида ацетата [11].
В ретроспективном когортном исследовании Bar-Hava и соавт. (2016) изучали эффективность интраназального аГнРГ для поддержки ЛФ у 46 пациенток с выраженным овариальным ответом на гонадотропную стимуляцию и заменой триггера овуляции на аГнРГ в программе ЭКО. Для поддержки ЛФ они использовали только нафарелин интраназально в течение 2 недель без микронизированного прогестерона. В результате были отмечены высокий уровень прогестерона в середине ЛФ и в день констатации беременности (190 рмоль/л). Частота клинической беременности составила 52,1% [26].
Особого внимания заслуживают результаты другого недавнего ретроспективного когортного исследования Bar-Hava (2017), в котором проанализированы результаты 2529 циклов ЭКО в протоколах с антГнРГ у 1479 пациенток, которые в зависимости от поддержки ЛФ были стратифицированы на 2 группы: в 1436 циклах пациентки получали аГнРГ интраназально (нафарелин 200 мг 2 раза в день) в течение 2 недель как единственный метод поддержки ЛФ, а в 1093 циклах – микронизированный прогестерон (8% гель по 1 аппликации 2 раза вдень) вагинально. Результаты исследования показали, что частота беременности и самопроизвольных выкидышей существенно не отличалась в обеих группах, однако в группе с аГнРГ отмечена более высокая частота родов (ОШ 1,59, 95% ДИ 1,07–2,36). Авторы предлагают использовать аГнРГ как единственный метод поддержки ЛФ в качестве достойной альтернативы микронизированному прогестерону [27].
Для каких пациенток комбинированный режим поддержки ЛФ с использованием аГнРГ наиболее предпочтителен – до сих пор не ясно. В РКИ Zafardoust и соавт. (2015) оценивали влияние добавления однократной дозы аГнРГ к микронизированному прогестерону на исходы программы ЭКО/ИКСИ в протоколах с антГнРГ у 100 женщин с повторными неудачами имплантации, из них 43 женщины получали 0,1 мг декапептила подкожно на 6-й день после ТВП, а 40 пациенток не получали аГнРГ. Оценивали частоту имплантации и частоту клинической беременности. Хотя возраст женщин, число и качество перенесенных эмбрионов были сопоставимы в обеих группах, отмечена более высокая частота имплантации (p=0,041) и частота клинической беременности (32,6% против 12,5%, p=0,030, OР=3,3, 95% ДИ 1,08–10,4) в группе пациенток с аГнРГ в ЛФ. Авторы пришли к заключению, что добавление к рутинной поддержке ЛФ микронизированным прогестероном агониста ГнРГ приводит к существенному повышению частоты имплантации и клинической беременности у женщин с повторными неудачами имплантации в анамнезе [23]. Другой категорией пациенток, которым можно рекомендовать аГнРГ для поддержки ЛФ, являются пациентки со снижением овариального резерва и предполагаемым «бедным» овариальным ответом на стимуляцию. Kung и соавт. (2014) ретроспективно проанализировали эффективность программы ЭКО/ИКСИ у 348 женщин, 240 из которых были в возрасте ≤38 лет и имели не более 2 неудач ВРТ в анамнезе. Из них 147 пациенток использовали в качестве дополнительной поддержки ЛФ декапептил на 6-й день после ТВП, а 93 женщины группы сравнения не использовали аГнРГ. Проведен подгрупповой анализ с учетом возраста, числа предшествующих попыток ВРТ, высокого уровня ФСГ и низкого числа полученных ооцитов. Поддержка ЛФ с добавлением декапептила повышала частоту имплантации (24,5% против 17,0%, p=0,023), частоту клинической беременности (49,0%, n=72 против 33,3%, n=31, p=0,023) и частоту родов живым плодом (41,5%, n=61 против 28,0%, n=26, p=0,039). Подгрупповой анализ показал повышение частоты клинической беременности при использовании аГнРГ в ЛФ у пациенток с уровнем ФСГ >8 mIU/mL (50,0%, n=15 против 8,3%, n=1, p=0,031). У пациенток с бедным овариальным ответом при получении менее 3 зрелых ооцитов выявлена более высокая частота клинической беременности (42,3%, n=11 против 0%, n=0, p=0,017) и частота родов живым плодом (30,8%, n=8 против 0%, n=0, p=0,035) [28]. В нашем исследовании при анализе исходов программы ЭКО у женщин, имеющих ≤2 неудач имплантации в анамнезе, добавление аГнРГ к рутинной поддержке ЛФ существенно повышало частоту клинической беременности (р=0,03). Таким образом, пациентки со снижением овариального резерва и повторными неудачами имплантации в программах ВРТ, по-видимому, являются оптимальной целевой категорией для использования комбинированного режима поддержки ЛФ (аГнРГ + микронизированный прогестерон).
Поскольку аГнРГ используют в период после переноса эмбрионов, закономерно возникает вопрос о возможном негативном влиянии аГнРГ на эмбрион/плод, риске неблагоприятных перинатальных исходов и врожденных пороков развития плода, о которых очень мало информации. Проведенные преклинические токсикологические исследования на животных не выявили каких-либо тератогенных эффектов аГнРГ [29]. До 1998 г. более 340 непланируемых спонтанных беременностей наступили после использования аГнРГ в ЛФ. Из них врожденные аномалии плода отмечены в 2,5% , а потери беременности – в 15% случаев. Их частота была сопоставима с популяционными показателями при ЭКО (2,39% и 22% соответственно) [30–32]. Кроме того, следует отметить, что в депо аГнРГ, таких как декапептил 3,75 мг, рутинно назначаемых в длинных протоколах в течение многих лет, активный пептид аГнРГ обнаруживается в кровотоке в течение 7–9 недель после введения препарата [33, 34], то есть препарат имеет достаточно продолжительный период полужизни, обнаруживается в ЛФ в длинных протоколах и не обладает негативными эффектами.
Несмотря на небольшое количество клинических исследований о возможных неблагоприятных тератогенных эффектах аГнРГ, 3 систематических обзора свидетельствуют об их отсутствии [3–5].
Заключение
Таким образом, результаты проведенного исследования показали, что использование аГнРГ в сочетании с микронизированным прогестероном для поддержки ЛФ в оказывает более позитивный эффект на исходы программы ЭКО по сравнению со стандартным режимом поддержки ЛФ микронизированным прогестероном. Механизм действия аГнРГ в ЛФ стимулированных циклов, по-видимому, является многофакторным и может осуществляться на различных уровнях, воздействуя на желтое тело, эндометрий и эмбрион.
Отмеченное повышение частоты многоплодной беременности и частоты преждевременных родов у пациенток, использующих аГнРГ для поддержки ЛФ в программе ЭКО, свидетельствуют о необходимости совмещения этого режима поддержки ЛФ с селективным переносом одного эмбриона.
Необходимы дальнейшие исследования, которые позволили бы определить оптимальные дозы и продолжительность использования препаратов аГнРГ в ЛФ в программе ЭКО, а также выявить целевую категорию пациенток для использования этого режима поддержки ЛФ в программе ЭКО.



