Адекватное развитие плаценты является ключевым звеном поддержания и успешного течения беременности. Развитие плаценты сопровождается ремоделированием материнских спиральных артерий, что способствует увеличению кровоснабжения матки за счет развития низкорезистентного и низкоскоростного кровотока в плацентарном ложе. При плацентарной недостаточности наблюдается снижение инвазии трофобласта, неполное ремоделирование спиральных артерий и преждевременное поступление материнской крови в ворсинчатый трофобласт, что может вести к окислительному повреждению ворсинчатого дерева. Термин «плацентарная недостаточность» охватывает ряд состояний, при которых плацента адекватно не функционирует, однако в большинстве случаев имеют ввиду преэклампсию (ПЭ) и задержку роста плода (ЗРП) [1].
ПЭ и ЗРП являются основными причинами перинатальной смертности и заболеваемости [2, 3]. ПЭ поражает 3–7% беременностей, на ее долю приходится свыше 10% случаев материнской смертности в индустриально развитых странах [4–7]. В Великобритании ПЭ/эклампсия была второй наиболее распространенной прямой причиной материнской смертности, составляя 0,38 случая на 100 000 родильниц [6]. В России материнская смертность от ПЭ занимает четвертое место после экстрагенитальных заболеваний, кровотечений и акушерской эмболии и составляет 1,16 на 100 000 родившихся живыми [8]. Приблизительно 20–30% беременностей с ПЭ ассоциируются с ЗРП [9]. Около 1/3 случаев ЗРП ассоциируются с ПЭ; доля их поднимается, когда фокусируются на заболевании с ранним началом [10].
ЗРП определяется, как неспособность плодом достичь генетического потенциала роста. При наиболее тяжелой форме ЗРП с ранним началом плод может погибнуть до достижения срока жизнеспособности или, если он родился крайне недоношенным, ребенок будет страдать от сопутствующей патологии [2]. ЗРП с поздним началом более коварна и трудно поддается определению; она также может приводить к антенатальной гибели, быть ассоциированной с дистрессом плода в родах и неонатальной энцефалопатией. Малый вес при рождении у выживших новорожденных повышает риск развития в дальнейшем неблагоприятных исходов, связанных сердечно-сосудистыми заболеваниями, формированием метаболического синдрома [11].
ПЭ характеризуется вновь возникшей гипертензией и протеинурией в сроки беременности ≥20 недель. При отсутствии протеинурии для постановки диагноза требуется наличие гипертензии вместе с подтвержденным системным заболеванием, таким как тромбоцитопения или повышенные уровни печеночных трансаминаз. Мультисистемные расстройства при ПЭ поражают различные органы, включая почки, печень и головной мозг.
Возможно, ПЭ и ЗРП являются различными манифестациями одного и того же заболевания, поскольку плацентарная патология при них имеет черты сходства [12]. К патогенетическим механизмам, вовлеченным в развитие ПЭ, относятся: нарушение глубокой плацентации, оксидативный стресс и эндоплазматический ретикулярный стресс, аутоантитела к рецептору 2-го типа ангиотензина II, активация тромбоцитов и тромбина, внутрисосудистое воспаление, эндотелиальная дисфункция и наличие антиангиогенного состояния, среди которых дисбаланс ангиогенеза рассматривается как один из наиболее важных факторов. Однако данный дисбаланс не является специфическим для ПЭ, поскольку он также возникает при ЗРП, гибели плода, спонтанной преждевременной родовой деятельности и инфарктах плаценты. Тяжесть и продолжительность ангиогенного дисбаланса вместе с восприимчивостью матери могут определять клиническое проявление ПЭ [13].
ПЭ может быть классифицирована, как ранняя (<34 недели) или поздняя (≥34 недели), в соответствии со сроком беременности на момент диагноза или родоразрешения [14, 15]. Хотя предлагаются некоторые другие границы гестационного возраста (такие, как 36 недель или преждевременная ПЭ и срочная ПЭ). Остается неясным, имеют ли ранняя/преждевременная и поздняя/срочная ПЭ различные патогенетические механизмы или же речь идет о градациях одного и того же лежащего в основе состояния [13, 16].
На протяжении последних десятилетий в фокусе исследований, посвященных пренатальной диагностике и скринингу, был плод и его врожденные структурные или генетические аномалии. Лишь относительно недавно, с приходом понимания того, что акушерские осложнения, ухудшающие перинатальные исходы, являются следствием патологических процессов в плаценте, в фокусе внимания совершенно новой области пренатального скрининга и диагностики оказалась плацента, ее здоровье, патологические плацентарные фенотипы [17]. Обширные исследования выявили ряд ранних эхографических, биохимических маркеров нарушенной плацентации [18]. Ряд индивидуальных клинических факторов, параметры допплеровской эхографии, сывороточные маркеры, были оценены в качестве предикторов ПЭ и/или ЗРП. Изучение протеома, метаболома, внеклеточных нуклеиновых кислот в материнской крови и других жидкостях является интригующей новой областью неинвазивного пренатального скрининга [1]. Разработка комплексного алгоритма оценки риска ПЭ и/или ЗРП уже в первом триместре беременности привлекает внимание в связи с возможностью назначения в сроки до 16 недель эффективных терапевтических вмешательств для профилактики данных осложнений [19]. Далее обсуждаются достигнутые за последнее время результаты в раннем прогнозировании и профилактике ПЭ и ЗРП, расстройств, ассоциированных с нарушением плацентации.
Эхографические маркеры патологической плацентации
Несмотря на множество важных функций, которые выполняет плацента с момента наступления беременности, традиционно плаценте и ее развитию, как это не странно, уделялось мало внимания и лишь недавно, с появлением новых технологий некоторые эхографические параметры были предложены для использования в прогнозировании осложнений беременности, связанных с патологической плацентацией.
Допплерометрия маточных артерий
Повышенная резистентность маточных артерий отражает недостаточность инвазии трофобласта в спиральные артерии и ассоциируется с развитием ПЭ и ЗРП. Резистентность сосудистому кровотоку может быть измерена неинвазивно в первом триместре беременности с помощью допплеровской оценки кровотока в маточном сегменте артерий, снабжающих спиральные артериолы.
Poon и соавт. (2009) после изучения данных 9149 одноплодных беременностей сообщили о частоте выявления ранней и поздней ПЭ по наименьшему из измеренных значений пульсационного индекса (ПИ), выраженному в MoM, 81,1% и 43,5% в левой и правой маточных артериях соответственно [20]. Demers и соавт. (2014) [21] отобрали для исследования 1810 женщин с ПЭ в анамнезе для оценки относительного риска повторного возникновения ПЭ, ЗРП при использовании среднего значения ПИ маточных артерий. Расчет ПИ в MoM был получен на основе анализа выборки, составившей 48500 пациенток. Для категории <34 недель относительный риск развития ПЭ составил 64,6. Не было выявлено значимых относительных рисков для группы >36 недель. Авторы пришли к выводу, что у женщин с ПЭ в анамнезе средние значения ПИ в маточных артериях в 11–13 недель обладают высокой прогностической силой для ранней и преждевременной ПЭ и ЗРП, но не для ПЭ при доношенном сроке беременности.

Мета-анализ Velauthar и соавт. (2014) [22], включивший 18 исследований с общей численностью почти 56 000 человек, оценил использование допплерометрии маточных артерий для прогнозирования ПЭ, ЗРП, мертворождения и отслойки плаценты в популяции низкого риска. Патологическим считался кровоток по данным допплерометрии маточных артерий при значениях индексов (ПИ и индекса резистентности) ≥90-й перцентили. Тест продемонстрировал относительно высокую специфичность, как для ПЭ с ранним началом (92,1%; 95% ДИ 88,6–94,6%), так и для ПЭ в целом (93,4%; 95% ДИ 90,4–95,5); его чувствительность была ограничена 47,8% (95% ДИ 39,0–-56,8%) для заболевания с ранним началом и 26,4% (95% ДИ 22,5–30,8%) – для ПЭ в целом. При использовании данного теста изолированно его прогностическая способность для клинического применения представлялась недостаточной. Использование допплерометрии маточных артерий для прогнозирования ЗРП на любом сроке беременности ассоциировалось с частотой выявления 15,4%, для ЗРП с ранним началом – 39,2% с частотой ложноположительного результата (ЛПР) 7%. [22]
В связи с различием рассматриваемых исходов, разными популяциями и разной частотой выявления и ЛПР сложно сравнивать данные представленных обзоров. ПИ маточных артерий демонстрирует обнадеживающие результаты, особенно по прогнозированию ПЭ и ЗРП с ранним началом.
Трехмерная эхография плаценты. Плацентарные сосудистые индексы
Оценка кривой скорости кровотока с помощью двухмерной (2D) допплерографии имеет ограниченную ценность в исследовании плацентарных нарушений при ПЭ и ЗРП. С другой стороны, трехмерная (3D) энергетическая допплерография предлагает новый подход к изучению плацентарной патофизиологии, способный дать информацию о васкуляризации плаценты и характеристике кровотока в ней. Данная техника может регистрировать характеристики внутриплацентарных сосудов, такие как изменение калибра, ветвление и перекруты. 3D-допплерометрия также может быть полезной в подтверждении успешности процесса инвазии трофобласта с помощью оценки дилатации спиральных артерий в их терминальных концах; также могут визуализироваться модификации сосудов, что характеризуется поступлением крови во внутриворсинчатое пространство [23]. Общее снижение плацентарной васкуляризации и нарушенное ветвление ворсинчатого кровотока предсказывает случаи ЗРП, не обнаруживаемые с помощью допплерометрии маточных и/или пупочных артерий. Индексы фракционного кровотока (VI, васкуляризационный индекс; FI, потоковый индекс; VFI, васкуляризационно-потоковый индекс) были изучены в нескольких работах, проводимых в первом триместре беременности.
Yu и соавт. [24] первыми оценили корреляцию фракционного объема крови через плаценту в зависимости от срока беременности. Значимость индексов была ниже, чем ожидалось при ПЭ и ЗРП [25] в сравнении с группой контроля, однако результаты по прогнозированию неблагоприятных последствий были достаточно невысокими, что могло быть следствием небольшого размера выборки. Noguchi и соавт. [26] в 2009 году изучили около 200 случаев с целью установления референсных значений индексов VI, FI и VFI для каждого гестационного срока с 12 до 40 недель. Ими также было проведено сравнение референсных значений с результатами, полученными в 13 случаях развития ЗРП. VI и VFI были расценены, как наилучшие маркеры при значениях ниже 1,5 стандартных отклонений от ожидаемых значений для случаев с ЗРП. Похожие результаты были получены в ретроспективном исследовании, в ходе которого наблюдалось 277 женщин в сроки беременности 10-13 недель: среди 24 случаев последующего развития ПЭ частота выявления данной патологии составила около 30% для всех трех индексов (VI, FI и VFI) [27]. Pomorski и соавт. [28] в 2011 году сообщили о частоте выявления ЗРП 60% для VFI, что, само по себе, является наилучшим параметром вслед за VI (50%) и FI (40%; частота выявления визуально подтверждена с помощью характеристической ROC-кривой).
В исследовании Hafner и соавт. [29] успешно использовался показатель VI плацентарного ложа для прогнозирования некоторых осложнений беременности, таких как ПЭ, в выборке, превышающей 4000 случаев. В первом триместре значения VI ниже 10-й перцентили для нормально протекающей беременности выявляли 60% случаев тяжелой ПЭ и 66,2% случаев ПЭ, сопровождающейся синдромом ЗРП. Более того, мультивариантный сравнительный анализ (который включал учет индекса массы тела и паритета) с допплерометрией маточных артерий, выполненный во втором триместре показал, что VI дает более высокую частоту выявления (80 против 60%). Авторы пришли к выводу, что VI может быть использован в качестве быстрой и надежной оценки риска тяжелых осложнений беременности в первом триместре.
Хотя данная технология показала многообещающие возможности, отсутствуют крупномасштабные исследования, которые бы валидировали ее использование. Кроме того, следует учитывать технические и методологические особенности широкого применения данного метода. Для изучения пользы данного инструмента в прогнозировании неблагоприятных исходов беременности, включая ПЭ и ЗРП, в первом триместре проводятся исследования, использующие стандартизованные подходы.
Биохимические и протеомные маркеры патологической плацентации
Материнские сывороточные маркеры являются минимально инвазивным тестом плодовой и плацентарной эндокринной функции, а также эндотелиальной дисфункции. Существующее понимание патофизиологии ПЭ и ЗРП дает основу для таких скрининговых тестов.
Большое количество биохимических маркеров было оценено для прогнозирования ПЭ (табл. 1). Два материнских сывороточных маркера плазменный протеин ассоциированный с беременностью (PAPP-A) и плацентарный фактор роста (PlGF) были подробно изучены и показали обнадеживающие результаты в плане прогнозирования ПЭ. Оба маркера продемонстрировали свою пользу при скрининге на анеуплоидии в 11–13 недель беременности и в настоящее время являются частью платформы автоматического определения, которая обеспечивает выдачу результатов в течение 30–40 мин после взятия образца [30]. Продолжаются исследования, призванные выявить альтернативные биомаркеры ПЭ.
При использовании протеомных подходов была обнаружена различная экспрессия множества новых классов белков при беременностях с ПЭ, в частности по экспрессии белков в первом триместре, включая плацентарные, сосудистые, транспортные, матриксные белки и белки острой фазы. Потенциальная ценность добавления матриксной металлопротеиназы (MMP) 9 в скрининговый алгоритм первого триместра на ПЭ, был оценен Poon и соавт. [31], однако не показал дополнительной ценности. Другие маркеры воспаления, такие как интерлейкин-1β повышались при ранней ПЭ [32]. Были опубликованы данные о белковых профилях (сигнатуре), прогнозирующих ЗРП на основе масс-спектрометрии, демонстрируя наиболее сильные ассоциации с аполипоптротеином С-II и С-III0 [33]. Эти исследования находятся в стадии разработки, однако существует огромный потенциал по улучшению интегрированных скрининговых инструментов за счет более высокой пропускной способности и эффективности подходов.
Нуклеиновые кислоты в материнской плазме и здоровье плаценты
Идентификация и/или количественная оценка внеклеточных нуклеиновых кислот быстро становится первичной скрининговой техникой для хромосомных и изолированных генных расстройств. Эта технология могла бы быть потенциально расширена за счет измерения экспрессии mRNA и/или microRNA, которые могут быть репрезентативными для оценки плацентарной функции [34]. Эти маркеры могут отражать изменения в материнском компартменте в качестве компенсации или ответа на плацентарную патологию или изменения, непосредственно выявленные при экспрессии плодовых/плацентарных генов (измерение плодовых или плацента-специфических внеклеточных нуклеиновых кислот). Эта захватывающая область исследований еще не достигла этапа клинического внедрения. Существуют немногочисленные сведения в отношении первого триместра, включая образцы материнской крови, взятой до развития клинических проявлений заболевания. Экспрессия некоторых microRNAs (miR-1233, miR-20, miR-144, miR-517-5p, miR-518b и miR-520h) показала изменения при беременностях с ПЭ [35]. В другом исследовании, сосредоточенном на количественной оценке C19MC miRNAs, которая считается плацента-специфичной, наблюдались изменения miRNA, связанные с ранней ПЭ, но не связанные с поздним развитием ЗРП. [36] Требуется проведение дальнейших исследований для подтверждения данных находок в других популяционных группах.
Комбинированные тесты
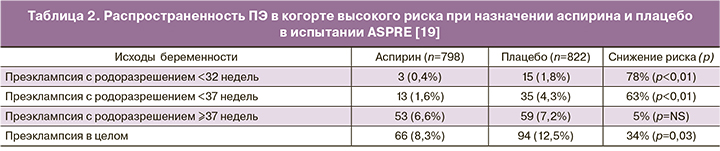
Было разработано несколько алгоритмов первого триместра по прогнозированию ПЭ с ранним началом (родоразрешение до 34 недель), хотя не все они были валидированы в независимых популяциях [18]. Один из алгоритмов, включающий оценку материнских характеристик, биофизических маркеров, таких как среднее артериальное давление и ПИ маточных артерий, а также биохимических маркеров PAPP-A и PlGF, был успешно валидирован во многих независимых популяциях [19, 37–39]. Он показал, что 89% случаев ПЭ до 32 недель, 75% случаев до 37 недель и 47% случаев >37 недель могут быть выявлены при фиксированной 10% частоте ЛПР [37]. Этот алгоритм затем был использован в качестве основы для определения риска в неотобранной популяции, в которой скрининг осуществлялся в 13 Европейских центрах в рамках многоцентрового испытания, оценивающего эффективность аспирина (150 мг перорально на ночь) для профилактики ПЭ [19]. Испытание ASPRE включало данные о 26 941 пациентах, в том числе 1776 женщин, относимых к группе высокого риска, которые были рандомизированы для профилактического назначения аспирина (n=878) или плацебо (n=898). Испытание показало значимое снижение – на 63% ПЭ, требующей родоразрешения до 37 недель (табл. 2) [19]. Распространенность заболевания, приводящего к очень раннему (<34 недель) родоразрешению снижалась на 78%. Распространенность заболевания с родоразрешением в сроки ≥37 недель была незначимо подвержена влиянию профилактической программы. Более того, авторы сообщили о 34% снижении ПЭ благодаря эффективному скринингу и профилактике. Терапия была более эффективной, когда приверженность ей превышала 90%, что служит аргументом в поддержку скрининга до назначения профилактики, а не просто универсальную профилактику. Вторичный анализ данных показал, что женщины с диагнозом хроническая гипертензия, демонстрировали склонность к резистентности профилактической терапии аспирином [40].
Хотя успех от стратегии прогнозирования и профилактики ранней ПЭ варьировал, предотвращая значимое число преждевременных родов, 75% заболевания возникало в сроки ≥37 недель [41]. Ни скрининговая программа (чувствительность 47%), ни профилактические вмешательства (5% незначимое снижение распространенности) не представляются особенно эффективными для данной когорты женщин. Наблюдается нарастающее количество данных в поддержку различных путей, приводящих к развитию ранних и поздних форм заболевания [42]. Существующая скрининговая стратегия, включающая допплеровскую оценку кровотока в маточных артериях и определение биохимических маркеров PAPP-A и PlGF, фокусируется вокруг плацентарной функции. Аспирин, который исходно назначался на основе предотвращения активации тромбоцитов и гиперкоагуляции в плаценте, также оказывает влияние на ангиогенез (PlGF) и плацентацию. Дальнейшее совершенствование скрининговой стратегии и прогнозирование ПЭ с поздним началом, вероятно, потребует включения маркеров эндотелиальной дисфункции. К проспективным инструментам относится измерение воспалительных маркеров, таких как hsCRP, оценка диаметра и кровотока в артерии сетчатки, а также оценка толщины интимы каротидной артерии. Однако ни один из упомянутых маркеров на данный момент не был включен в комбинированную модель и валидирован в скрининговых алгоритмах [43].
«Порог» эффективности скрининга для ЗРП был установлен Poon и соавт. (2013) [44], которые сообщили, что при 55,5% преждевременных и в 44,3% срочных родах плоды имели вес более 5-й процентили, который мог быть выявлен при использовании таких же инструментов исследования, что и описанные для скрининга ПЭ. Тест основывался на оценке материнских характеристик, допплерометрии маточных артерий и артериального давления матери, а также биохимических маркеров PAPP-A и PlGF. В отличие от работы, проведенной той же группой исследователей для скрининга ПЭ, отсутствуют исследования, которые бы валидировали данный алгоритм в других популяциях. Потенциальное терапевтическое вмешательство для ранней ЗРП (заболевание, связанное с плацентарной недостаточностью) аналогично таковому для ранней ПЭ, а именно – назначение аспирина. Значимость аспирина для профилактики ЗРП была оценена в многолетнем мета-анализе, хотя все еще не существует крупных исследований, связывающих прогнозирование ЗРП в первом триместре с успешным терапевтическим вмешательством.
Другие исследовательские группы, наиболее заметной из которых является команда из Барселоны, также фокусировались на разработке прогностических моделей ЗРП в первом триместре [10]. Этот алгоритм имел сходный формат, включая материнские характеристики, биофизические параметры и биохимические маркеры, хотя биохимическая панель различалась, включая растворимую fms-подобную тирозинкиназу (sFlt) 1, а не PAPP-A. Определения исходов, использованные данной исследовательской группой, также различались – определение ранней и поздней ЗРП с границей отсечки 34 недели и с использованием различных определений ЗРП. Данная группа сообщила о частоте выявления 86,4% (при 10% частоте ЛПР) для ранней ЗРП. [10] Частота выявления для поздней ЗРП составила 65,8%. Область под ROC-кривой для скрининга составила 0,93 и 0,76 соответственно. Данная многообещающая скрининговая модель также требует проведения внешней валидации. Авторы продолжали наблюдать значимость повторных измерений, оценивая допплерометрию маточных артерий и уровни PlGF, как в первом, так и во втором триместрах беременности, но не выявили более высокой ценности, чем при изолированной оценке в первом или втором триместрах [45].
Ценность sFlt при скрининге в первом триместре поддерживается другими данными. Inan и соавт. [46]
показали, что Prokineticin-1 (также описанный, как эндокринный железистый сосудисто-эндотелиальный фактор роста (VEGF)) повышается при ЗРП (85,7% чувствительности при 72,5% специфичности). Takenaka и соавт. [47] показали, что уровни mRNA рецептора 1 VEGF в первом триместре значимо повышаются при беременностях с ЗРП (среднее 2,63 (стандартное отклонение 0,34) против 2,18 (0,54); p=0,01). Эхографическая оценка объема плаценты не представляется столь же ценным маркером (25% чувствительности при 90% специфичности), и биохимическая оценка плацентарной функции представляется предпочтительной [48]. Добавление других биофизических измерений, таких как измерение ПИ в глазной артерии, по-видимому, не повышает эффективность допплеровской оценки маточных артерий [49]. Последней интересной находкой является то, что уровни Lipoxin A2 снижаются при беременностях, при которых развивается ЗРП. Lipoxin A2 модулирует воспалительный ответ и играет противовоспалительную и проангиогенную роль в процессе плацентации [50]. Также интересно заметить, что аспирин может оказывать влияние на метаболизм Lipoxin А2.
Заключение
Наблюдается значительный прогресс в раннем прогнозировании ПЭ и ЗРП, распространенных неблагоприятных исходов беременности, ассоциированных с патологической плацентацией и плацентарной дисфункцией. Допплерометрия маточных артерий является наиболее изученным маркером с хорошим прогностическим потенциалом, может легко интегрироваться с другими неэхографическими маркерами. Сосудистые индексы, рассчитанные при использовании 3D-эхографического картирования плаценты и плацентарного ложа, являются перспективным инструментом скрининга на ЗРП и ПЭ, однако требуют проведения дальнейшей оценки их роли в клинической практике. Многообещающим является оценка ангиогенных факторов (PlGF и sFlt1) и их роли в раннем прогнозировании ПЭ и ЗРП. В то же время идея идентификации единственного маркера или панели маркеров, способных прогнозировать все случаи ПЭ и ЗРП представляется мало реалистичной, поскольку к идентичным исходам может вести множество этиологических факторов.
Для прогнозирования ПЭ в настоящее время доступны надежные комбинированные алгоритмы скрининга в первом триместре, которые были валидированы на различных популяциях. Это имеет особое значение, поскольку вмешательства с целью профилактики преждевременной (<37 нед) ПЭ приводят к значимому снижению распространенности заболевания, если сочетаются с программой прогнозирования риска первого триместра. Алгоритмы для прогнозирования ЗРП менее эффективны. Сравнение подходов часто затруднительно, поскольку исследователи часто используют различные критерии определения задержки роста. Алгоритмы, обычно представляющиеся менее эффективными, могут отражать различия в причинах состояния.
Потребуется дальнейшее развитие и комбинирование инструментов по оценке функции плаценты, что будет способствовать появлению направленных вмешательств с возможностью их терапевтического использования для персонализации профилактики и клинического ведения при ПЭ и ЗРП.



