Внутриматочные синехии представляют собой состояние, характеризующееся нарушением анатомической целостности полости матки за счет образования спаек разной степени выраженности. В последние десятилетия в связи со значительным повышением частоты внутриматочных вмешательств, распространенность внутриматочных синехий увеличилась, составляя, по данным разных авторов от 0,3 до 21,5% [1–5]. Несмотря на множество предложенных вариантов лечения и профилактики, высока вероятность рецидива данного состояния, в особенности при тяжелой степени, характеризующейся значительной облитерацией полости матки. Процессы фиброза эндометрия, происходящие при внутриматочных синехиях, характеризуются избыточным осаждением и реорганизацией внеклеточного матрикса (ВКМ), который «замещает» здоровый эндометрий [3]. Результаты биопсии эндометрия при внутриматочных синехиях подтвердили наличие 50–80% фиброзной ткани, когда как содержание фиброзной ткани в эндометрии здоровых женщин составляло 13–20% [6]. Публикации последних лет немаловажную роль в формировании фиброзной ткани отводят процессам гипоксии, пониженной неоваскуляризации, аномальной экспрессии цитокинов, нарушению взаимодействия в сигнальных путях [2, 7, 8]. Однако, несмотря на наличие большого количества проведенных исследований, механизм формирования внутриматочных синехий, необходимый для создания новых методов таргетной терапии и профилактики, недостаточно изучен.
Современные технологии – микроматричный анализ и высокопроизводительное секвенирование – позволяют оценить транскрипционную активность широкого спектра генов. Предложенные технологии стали основой нескольких десятков публикаций в литературе, основанных на различии транскрипционных профилей генов при том или ином заболевании. Так, обнаружены гены-кандидаты патофизиологических изменений, происходящих в эндометрии пациенток при эндометриозе, преэклампсии, раке яичников [9–11]. Принимая во внимание результаты этих публикаций, целью нашего исследования стало выявление генов, экспрессия которых отличается у пациенток с внутриматочными синехиями, по сравнению с эндометрием условно здоровых женщин.
Материал и методы исследования
На базе отделения оперативной гинекологии и лаборатории молекулярно-генетических методов ФГБУ Национальный медицинский исследовательский центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России проведено проспективное исследование 20 пациенток репродуктивного возраста, которые прошли полное клинико-лабораторное обследование и лечение в 2015–2016 гг. Все пациентки перед включением в исследование подписали добровольное информированное согласие. Исследование было одобрено комитетом по этике ФГБУ НМИЦ АГП им. В.И. Кулакова Минздрава России. Пациентки были разделены на 2 группы: 1-я группа – женщины с внутриматочными синехиями; 2-я группа – условно здоровые женщины. Критериями включения в исследование были: возраст пациенток от 18 до 45 лет, наличие внутриматочных синехий, выявленных с использованием инструментальных методов обследования и подтвержденных гистологическим исследованием, пролиферативная фаза менструального цикла; отсутствие патологических изменений в эндометрии по результатам гистологического исследования. Критериями исключения из исследования были: возраст пациенток менее 18 и больше 45 лет, прием гормональных препаратов на момент обращения, тяжелая сопутствующая соматическая патология, злокачественные новообразования, острые воспалительные заболевания органов малого таза. Гистероскопический этап выполняли по стандартной методике в отделении оперативной гинекологии в условиях соответствующего анестезиологического обеспечения.
В качестве материала для исследования были использованы образцы пролиферативного эндометрия, взятые в предоперационном периоде при помощи пайпель-биопсии в контейнеры с транспортной средой «Стор-экс» («ДНК-технология», Россия) до гистероскопии с последующим диагностическим выскабливанием эндометрия и гистологическим исследованием. Хранение образцов эндометрия производилось в биобанке ФГБУ НМИЦ АГП им. В.И. Кулакова Минздрава России при температуре -70°C.
Процедура выделения 500 нг РНК каждого образца с использованием набора RNeasy Mini Kit (Qiagen, США) проводилась по стандартной методике с предварительным синтезированием целевой кДНК с помощью транскрипции с последующей фрагментацией образцов согласно протоколу производителя (Affymetrix, США). Для гибридизации образцов использовались микрочипы GeneChip Human Exon 1.0 ST Arrays (Affymetrix, США) с экспозицией 60 об./мин в течение 17 часов при 45°C. Промывка и окрашивание микрочипов осуществлялись на Fluidic Station 450 (Affymetrix, США), сканирование – на сканере Affymetrix GeneChip 3000 7G (Affymetrix, США). Для получения файлов-изображений DAT микромассивов использовался пакет программ Affymetrix GeneChip Command Console (version 0.0.0.676, Affymetrix). Полученные данные были проанализированы с использованием программ Expression Console и TAC (Transcriptone Analysis Console) (Affymetrix, США).
Был проведен дополнительный биоинформатический анализ данных, полученных с помощью микроматричного анализа. Выявленные гены были проанализированы на предмет участия в типовых биологических процессах (pathways) с помощью сервиса Gene Ontology [9, 10]. Данный сервис описывает широкий перечень генов, их ключевых продуктов и свойств, а также составляет стандартизованное описание биологических функций и процессов с перечислением задействованных компонентов, благодаря чему возможно выявлять общие процессы, в которых задействованы интересующие гены и их группы.
Статистическая обработка данных выполнена с помощью пакета прикладных программ SPSS Statistics 17.0. Для оценки значимости межгрупповых различий применяли тест Стьюдента для двух независимых выборок. При исследовании двух выборок использовали критерий Манна–Уитни для несвязанных совокупностей. Статистически значимыми считались различия при p<0,05.
Результаты исследования
Основная группа была представлена 10 пациентками с внутриматочными синехиями, степень тяжести которых соответствовала II (50%) и III (50%) по классификации Американского общества фертильности [6].
Анализ возрастных характеристик пациенток, включенных в исследование, статистически значимых различий не выявил. Средний возраст женщин в 1-й группе составил 33,67±5,48 года, во 2-й группе – 32,00±5,14 года. У всех женщин, включенных в исследование, был регулярный менструальный цикл: возраст наступления менархе в 1-й группе – 13,1±1,4 года, во 2-й группе – 12,5±1,3 года. Длительность менструального цикла составляла от 5 до 7 дней, средняя продолжительность менструального цикла в 1-й группе была 29,1±3,5 дня, во 2-й группе – 30,5±3,1 дня.
Пациентки в исследуемых группах были сопоставимы по частоте гинекологических и экстрагенитальных заболеваний. Анализ перенесенных оперативных вмешательств выявил, что наиболее часто в основной группе были проведены гистероскопия и диагностическое выскабливание – 40% (n=4). Оценка репродуктивной функции выявила преобладание вторичного бесплодия в основной группе по сравнению с группой контроля – у 6 (60%) и 2 (20%) пациенток соответственно.
В ходе проведенного исследования при сравнении экспрессии генов эндометрия в фазу пролиферации у женщин основной группы и группы сравнения установлено, что в группе женщин с внутриматочными синехиями наблюдалось повышение уровня мРНК 9 генов и понижение уровня мРНК 2 генов (табл. 1). Результаты иерархического кластерного анализа по 30 различно экспрессирующимся генам представлены на рисунке.
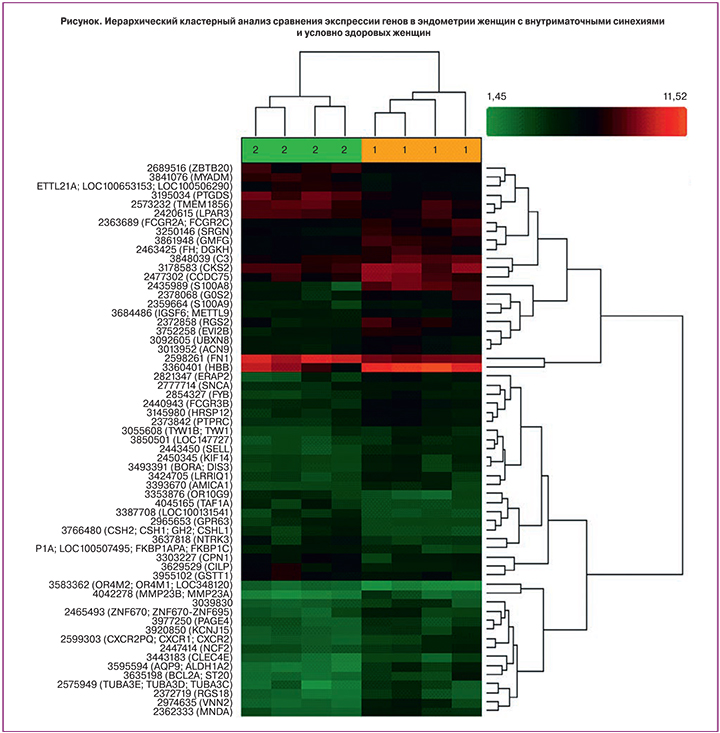
Таким образом, в результате сравнительного анализа выявлены различия в уровнях мРНК следующих генов: S100 calcium binding protein A8 (S100A8) – повышение в 10,94 раза (p=0,000443), hemoglobin beta (HBB) – повышение в 5,53 раза (р=0,020497), vanin 2 (VNN2) – повышение в 5,1 раза (р=0,004938), regulator of G-protein signaling 2, 24kDa (RGS2) – повышение в 4,97 раза (р=0,007148), endoplasmic reticulum aminopeptidase 2 (ERAP2) – повышение в 4,69 раза (р=0,008723), aquaporin 9, aldehyde dehydrogenase 1 family, member A (AQP9) – повышение в 4,67 раза (р=0,000299), myeloid cell nuclear differentiation antigen (MNDA) – повышение в 3,81 раза (р=0,001250), tubulin, alpha 3e (TUBA3E) – повышение в 3,58 раза (р=0,016996), Fc fragment of IgG, low affinity IIIb, receptor (FCGR3B) – повышение в 3,03 раза (р=0,000820), prostaglandin D2 synthase 21 kDa (PTGDS) – понижение в 2,9 раза (р=0,001476), neurotrophic tyrosine kinase, receptor, type 3 (NTRK3) – понижение в 3,04 раза (р=0,040756)
Кластерный анализ генов, представленный на рисунке, выявил распределение по группам, которые значительно различаются по уровню экспрессии генов.
Был проведен дополнительный биоинформатический анализ данных, полученных с помощью микроматричного анализа. Выявленные гены (табл. 2) были проанализированы на предмет участия в типовых биологических процессах (pathways) с помощью сервиса Gene Ontology [9, 10].
Анализ генов, представленность продуктов которых демонстрирует наибольшие отличия в биоптатах эндометрия женщин с внутриматочными синехиями, по сравнению с биоптатами эндометрия условно здоровых женщин, с помощью сервиса Gene Ontology выявил ряд биологических процессов, в которых задействованы некоторые из выявленных генов (табл. 2; представлены процессы, в которых задействовано 4 и более генов). Как можно видеть, большинство обнаруженных генов (6 из 9) задействованы в процессах иммунного ответа, в том числе в активации нейтрофилов, а также ряде других аспектов функционирования нейтрофилов и гранулоцитов. Кроме того, ряд генов (4 из 9) задействованы также в экзоцитозе. Стоит отметить, что эти перечни пересекаются, тем самым подтверждая роль межклеточного сигналинга, как одного из важнейших компонентов иммунного ответа. Экзоцитоз же представляет собой один из механизмов доставки сигнальных молекул на поверхность клетки и во внеклеточное пространство.

Обсуждение
Повышение частоты выявления внутриматочных синехий связано как с увеличением количества женщин позднего репродуктивного возраста, страдающих бесплодием, перенесших оперативные вмешательства, так и с расширением диагностических возможностей инструментальных методов исследования, в том числе с внедрением в алгоритм обследования гистероскопии.
Проведенное исследование подтвердило ведущую роль ятрогенного фактора в формировании внутриматочных синехий, что согласуется с результатами других исследований [3, 7]. Преобладание вторичного бесплодия над первичным у пациенток с внутриматочными синехиями подтверждает предположение о наибольшей вероятности формирования спаек после хирургических вмешательств в полости матки в анамнезе.
Внедрение альтернативных малоинвазивных методов диагностики с целью повышения результативности исходов комплексного лечения и профилактики заболевания является необходимым условием развития медицины и здравоохранения [7]. Для создания таких моделей диагностики необходимо знание основополагающих молекулярно-биологических процессов развития того или иного заболевания. Исследования последних лет подтверждают, что изучение состава генома, процессов экспрессии и транскрипции отдельных генов является обоснованным и необходимым условием при ряде заболеваний [11], что послужило основанием для изучения нами профиля экспрессии генов при внутриматочных синехиях. Полученные нами данные с применением высокопроизводительного секвенирования и гибридизации на чипах с использованием чипов Human Exon ST Array 2.0 при внутриматочных синехиях позволили выделить ряд генов, уровень мРНК которых значительно отличался от мРНК условно здоровых женщин: S100A8, HBB, VNN2, RGS2, ERAP2, AQP9, MNDA, FCGR3B, PTGDS, NTRK3.
Ген кальций-связывающего протеина (S100A8), также известный как кальпротектин, представляет собой антимикробный пептид, выявленный при процессах заживления ран у людей и мышей. Этот белок имеет известные функции в иммунном ответе и выступает в качестве молекулярного фрагмента, ассоциированного с повреждением, который сигнализирует через рецепторы о конечных продуктах гликирования, действуя как эндогенный лиганд для толл-подобного рецептора 4 (TLR4) [12]. Повышение экспрессии кальций-связывающего протеина выявлено при иммунной активации и воспалении при различных заболеваниях, в том числе при сахарном диабете, ожирении, сердечно-сосудистых заболеваниях, артрите, раке, панкреатите, псориазе, воспалительных заболеваниях кишечника и болезнетворных инфекциях [13]. S100A8 составляет структуру до 60% всех полиморфноядерных нейтрофилов, обладая антимикробной активностью в отношении Escherichia coli, Klebsiella и Staphylococcus spp. в концентрации 64–256 мг/л in vitro. В работе Trostrup и соавт. [14] отмечено выраженное антимикробное действие S100A8 в присутствии Pseudomonas aeruginosa, а также высокий регенеративный потенциал. Эта двойственность свойств позволила авторам рассматривать кальций-связывающий протеин в качестве компонента индивидуальной вспомогательной иммунотерапии в будущих исследованиях.
Ванин 2 (VNN2) – белок, состоящий из пептидных и гидрофобных областей, которые необходимы для соединения с гликозилфосфатидилинозитолом и последующего прикрепления к клеточной мембране. В тканях млекопитающих VNN2 является единственным известным источником активности пантотеиназы, которая участвует в гидролизе пантотеина для создания пантотеновой кислоты (также называемая витамин В5 или пантотенат) и цистамина. Функциональные исследования сообщают, что пантотеновая кислота играет важную роль в защите клеток от окислительного повреждения за счет увеличения содержания глутатиона [15]. Она является важнейшим субстратом для синтеза коэнзима А – кофактора, участвующего в нескольких биологических процессах, таких как синтез жирных кислот, необходимых для производства стероидных гормонов и окисления пирувата в качестве топлива для цикла лимонной кислоты [16]. Цистамин является мощным антиоксидантом, который защищает клетки от окислительного стресса при сахарном диабете 1-го типа в островковых клетках. VNN1 также способствует поддержанию воспаления частично путем ингибирования пролиферации пероксисом-тиазолидиндионом активированного рецептора-с, тем самым вызывая увеличение экспрессии цитокинов и хемокинов при воспалительной реакции [16, 17]. Кроме того, отмечена функциональная роль VNN1 в регуляции клеточной адгезии в процессе миграции гемопоэтических клеток в тимус, а VNN2 был вовлечен в адгезию тромбоцитов и миграцию в лимфатических узлах. В работе Sayasith и соавт. отмечено экспериментальное гонадотропин-зависимое повышение экспрессии VNN2 в фолликулярной жидкости коров в предовуляторную фазу, что рассматривается авторами как защитная реакция клеток гранулезы на предшествующий «воспалительный фон» процесса овуляции [15].
Регулятор передачи сигналов G-белка 2 (RGS2) представлен доменом, который активирует гуанозин-трифосфатазу через α-субъединицу белок-связывающего рецептора G и тем самым влияет на скорость его дезактивации [18]. Дефицит RGS2 у мышей предполагал нормальную фертильность. Экспрессия RGS2 в клетках гранулезы крыс была вызвана введением хорионического гонадотропина человека, а регуляция RGS2 в клетках гранулезы мышей и женщин подавлялась транскрипцией 2 субъединицы цитохромоксидазы (COX2) – таргетного гена, который снижает концентрацию хорионического гонадотропина человека [19]. Анализ на микрочипах выявил, что Rgs2 является одним из генов, который регулируется гонадотропин-рилизинг-гормоном. Экспрессия RGS2 в фолликулярных клетках явилась результатом переноса эмбрионов, предполагая, что RGS представляет собой потенциальный биомаркер, ответственный за развитие яйцеклетки и пролонгирование беременности [19, 20]. Повышение экспрессии RGS2 в эндометриальных стромальных клетках было выявлено на протяжении всего периимплантационного периода и не зависело от наличия эмбриона и уровней эстрогенов и прогестерона, что позволило авторам предположить участие RGS2 в механизмах местной иммунорегуляции [20]. В исследовании Karppanen и соавт. выявлена ассоциация RGS2 с развитием преэклампсии у женщин с избыточной массой тела [21].
Аминопептидаза-2 эндоплазматического ретикулума (ERAP2) играет одну из важных ролей в функционировании главного комплекса гистосовместимости: создание пептидов нужной длины и последовательности из деградированных белков, проникших в эндоплазматический ретикулум, с последующей презентацией на своей поверхности [22]. Johnson и соавт. выявили, что ERAP2 ассоциирована с преэклампсией в этнических группах Австралии и Новой Зеландии, а также Норвегии [23]. В каждой из изученных ими групп обнаружены различные полиморфизмы генов. ERAP2 синтезируется синцитиотрофобластом и представляет собой член подсемейства окситоциназы аминопептидаз М1, которые играют важную роль в поддержании нормальной беременности [21, 24]. Кроме того, известна роль ERAP2 в регуляции артериального давления, продукции провоспалительных цитокинов, иммунном ответе [22, 25]. Измененная экспрессия ERAP2 в первом триместре беременности предполагает развитие преэклампсии в последующем [24].
Аквапорин 9 (AQP9) является неотъемлемым белком плазматической мембраны, который осуществляет функцию транспорта воды. Белки этого семейства были идентифицированы более 15 лет назад. Присутствие аквапоринов в женской репродуктивной системе было подтверждено опытами на мышах. AQP9 принимает участие в притоке воды в фолликулы яичника через механизмы трансцеллюлярного транспорта. Иммуногистохимический анализ выявил экспрессию AQP9 эпителиальными клетками маточных труб у мышей. В работе Skowronski уровень экспрессии AQP9 не менялся со 2–4-го по 10–12-й дни менструального цикла, однако отмечалось повышение экспрессии на 14–16-й и 18–20-й дни и на ранних сроках беременности [26]. Кроме того, повышение экспрессии AQP9 коррелировало с высокими значениями хорионического гонадотропина при преэклампсии [27].
Ядерный дифференцировочный антиген миелоидной клетки (MNDA) является представителем семейства интерферон-регулируемых генов, выявленных у человека и мышей. Экспрессия ограниченным паттерном гемопоэтических клеток стала основой для идентификации MNDA, что подтвердилось при изучении нормальных и опухолевых клеток и явилось уникальным свойством генов этого подсемейства. Кроме того, анализ выявил наличие MNDA в миелобластах и на более поздних стадиях развития миеломоноцитарной клетки, включая моноциты периферической крови и гранулоциты, в том числе на участках воспаления, за исключением гистиоцитов. Участие MNDA в регуляции экспрессии гомолога протонного белка (DLK1), позволило предположить, что этот антиген является модулятором транскрипции факторов, опосредующих апоптоз. Регулирование содержания MNDA в цитоплазме клеток при сепсисе в сторону снижения его концентрации по данным Milot и соавт. приводит к угнетению апоптоза, следовательно, это может стать предпосылкой для лечения сепсиса и других воспалительных процессов [28].
Фрагмент иммуноглобулина G с низкой чувствительностью к рецепторам 3B (FCGR3B) представляет собой ген, сгруппированный в хромосоме 1 локуса q23-24, проявляющий аллельный полиморфизм. Fc рецепторы Ig G играют важную роль медиаторов между гуморальной и клеточной регуляцией иммуного ответа [29]. Антитела к FcyRIIIb-полиморфным антигенам HNA1a, HNA1b, HNA1c участвуют в процессе аутоиммунной нейтропении, нейтропении у новорожденных, а также при остром повреждении легкого при переливании крови. Расположение Fc рецептора 3В в FCGR-гене кластера полиморфных генов подразумевает вовлечение его в задействование и активацию полиморфноядерных нейтрофилов к зонам воспаления. Кроме того, различные вариации FCGR3B ассоциированы с такими заболеваниями, как ревматоидный артрит, язвенный колит, гломерулонефрит [30].
Известная функция гемоглобина-β (HBB) – белка, транспортирующего кислород и углекислый газ на эритроцитах, в последнее десятилетие подвергается дискуссии. Помимо эритроцитов, была доказана продукция гемоглобина активированными макрофагами, клетками хрусталика глаза и альвеолярными эпителиальными клетками [31, 32], что в свою очередь послужило основой защитной функции HBB от окислительного стресса [33, 34]. Глобальный анализ экспрессии генов, проведенный Dassen и соавт., выявил, что в эндометрии женщин происходит выраженная экспрессия различных видов семейства гена гемоглобина (гемоглобинов А, B, D и G) [35]. Кроме того, выраженная экспрессия гемоглобина-β выявлена и в окно имплантации [36].
Гены с пониженным уровнем экспрессии в пролиферативном эндометрии у пациенток с внутриматочными синехиями, выявленные в нашем исследовании (PTGDS, NTRK3), отличались невысокими уровнями значений отклонения от нормы, что позволяет нам не рассматривать их в качестве потенциальных перспективных биомаркеров.
Данные, полученные в нашем исследовании с помощью дополнительного биоинформатического анализа, позволяют предположить, что одной из причин развития внутриматочных синехий являются отклонения в процессе межклеточного взаимодействия у таких пациенток. Возможно, это связано с внутриматочной инфекцией, ответ на которую приводит к развитию спаечного процесса. Также можно предположить, что у пациенток с внутриматочными синехиями отличаются параметры «тонкой настройки» иммунной системы на фоне внутриматочной инфекции и/или микротравм эндометрия, что приводит к патологическому процессу в эндометрии.
Следует отметить, что анализ различий в представленности транскриптов в ходе данной работы проведен для небольшого количества образцов. Следствием этого может являться неполное выявление всех действительно участвующих в процессе формирования синехий генов. В связи с этим требуется проведение дополнительного исследования на более широкой выборке.
Заключение
Таким образом, обобщая полученные нами данные об изменении экспрессии ряда генов в пролиферативном эндометрии женщин с внутриматочными синехиями, важно отметить взаимосвязь этих генов с нарушением процессов апоптоза, а также вовлеченность их в процессы воспаления, иммунного ответа и межклеточного взаимодействия. Данные об изменении экспрессии генов S100A8, HBB, VNN2, RGS2, ERAP2, AQP9, MNDA, TUBA3E, FSGR3B в пролиферативном эндометрии у женщин с внутриматочными синехиями, впервые полученные в нашем исследовании, являются перспективными для понимания патогенетических механизмов этого заболевания. Дальнейшие исследования в этом направлении позволят использовать генетические маркеры с целью создания малоинвазивных тестов для формирования групп риска и возможного прогнозирования степени тяжести процесса.



