Вирус папилломы человека (ВПЧ) широко распространен в окружающей среде и в популяции человека на всем пространстве Земли. Вызываемые им патологические процессы (остроконечные кондиломы, бородавки, заболевания шейки матки, включая рак) представляют серьезную угрозу здоровью и даже жизни женщин. Папилломавирусная инфекция является основным этиологическим фактором развития предрака и рака аногенитальной области [1]. Не только высокая распространенность ВПЧ в природе, но и улучшение методов диагностики показывают, что частота его обнаружения при отсутствии каких-либо симптомов у молодых женщин, составляет от 40 до 80%, а вероятность персистенции ВПЧ-инфекции – 80–90% [2]. Так, в США ежегодно у 6,2 млн человек выявляется инфекция ВПЧ [3]. Онкогенные типы ВПЧ ассоциированы прежде всего с плоскоклеточными интраэпителиальными поражениями шейки матки (цервикальная интраэпителиальная неоплазия – CIN), ануса – (анальная интраээпителиальная неоплазия – AIN), вульвы – (вульварная интраэпителиальная неоплазия – VIN), влагалища – (вагинальная интраэпителиальная неоплазия – VaIN) и злокачественными опухолями, рак шейки матки – 91%, вульвы – 69%, влагалища – 75%, ассоциированного рака среди женщин [1, 2].
Важная роль в профилактике рака шейки матки принадлежит ранней диагностики ВПЧ, особенно его онкогенных типов. Кроме того, известно, что развитие неоплазий шейки матки во многом зависит от состояния микробиоценоза влагалища. Ненормальная микрофлора влагалища и цервикального канала ассоциированы с возникновением предрака и рака шейки матки. В этой связи нами проведено исследование, цель которого заключалась в изучении возможностей раннего скрининга инфекций, передаваемых половым путем (ИППП), включая ВПЧ, валидации и сравнительном анализе современных тестовых наборов для самостоятельного взятия биоматериала из влагалища.
Материал и методы исследования
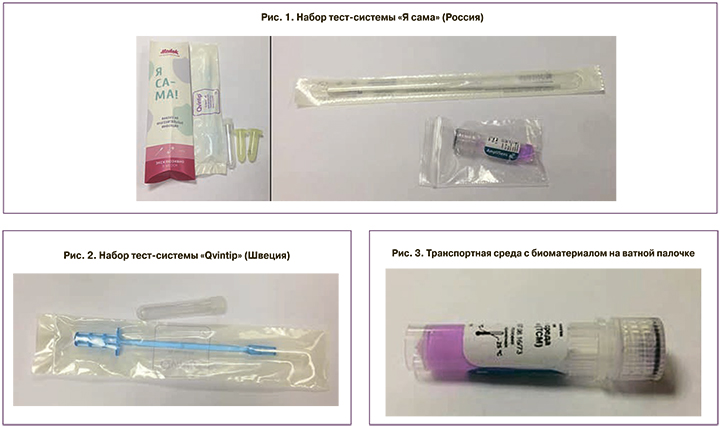
В период с 1 декабря 2016 г. по 30 декабря 2017 г. проведено многоцентровое простое изучение двух тестовых систем для самостоятельного взятия пациенткой биоматериала из влагалища, предназначенных для ранней диагностики ВПЧ (рис. 1, 2): 1-я тест-система «Я сама» (Россия) и 2-я – «Qvintip» (Швеция).
В набор «Я сама» входит ватный тампон и транспортная среда, в набор «Qvintip» – пластиковый аппликатор и пробирка без транспортной среды.
Для реализации поставленной цели нами проведено скрининговое обследование 110 женщин в возрасте от 21 до 45 лет (в среднем 29±0,6 года), не предъявляющих жалоб на женское здоровье. В анамнезе подавляющее большинство пациенток (102 из 110, или 92,7%) отмечали периодическое появление выделений из влагалища; у 71 (64,5%) женщины ранее выставляли диагноз вульво-вагинального кандидоза и/или бактериального вагиноза. Доброкачественные заболевания шейки матки в анамнезе отметили 58 из 110 обследованных (52,7%). Все 110 пациенток имели в жизни по крайней мере один эпизод лечения вагинальных инфекций с интравагинальным применением лекарственных средств или биоактивных добавок.
Критерии включения: подписанное добровольное согласие на участие в исследовании.
Критерии исключения: беременность, послеродовой период, лактация, психические заболевания, наркомания, алкоголизм, невозможность выполнения протокола.
Валидация систем проводилась по ниже перечисленным инфекциям:
- Chlamydia trachomatis (301уро);
- Neisseria gonorrhoeae (306 уро);
- Mycoplasma genitalium (308 уро);
- Trichomonas vaginalis (307 уро);
- Human papillomavirus, HPV (313С уро).
 Генотипирование 22 типов ВПЧ – 6, 11, 16, 18, 26, 31, 33, 35, 39, 44, 45, 51, 52, 53, 55, 56, 58, 59, 66, 68, 73, 82 с определением вирусной нагрузки осуществлялось методом мультиплексной полимеразно-цепной реакции с детекцией результатов в режиме реального времени (HPV квант-21, «ДНК-Технология», Россия) в цервикальном канале.
Генотипирование 22 типов ВПЧ – 6, 11, 16, 18, 26, 31, 33, 35, 39, 44, 45, 51, 52, 53, 55, 56, 58, 59, 66, 68, 73, 82 с определением вирусной нагрузки осуществлялось методом мультиплексной полимеразно-цепной реакции с детекцией результатов в режиме реального времени (HPV квант-21, «ДНК-Технология», Россия) в цервикальном канале.
Клиническое обследование пациенток включало традиционную оценку наличия или отсутствия жалоб, клинико-анамнестический сбор данных, физикальное обследование, клинический осмотр и определение гинекологического статуса; рН-метрию влагалищной жидкости (ВЖ).
Каждой женщине перед гинекологическим осмотром предлагалось самостоятельно как можно глубже (ближе к шейке матки) взять биоматериал из влагалища с помощью ватного тампона и шпателя. В первом случае ватную палочку с биоматериалом опускали в транспортную среду (пробирка с розовой транспортной средой системы «Я сама»), палочку отламывали и оставляли в пробирке (рис. 3).
При использовании системы «Qvintip» после взятия нативного материала из просвета влагалища аппликатор опускали в пробирку, сгибали и отсоединяли его от фиксатора, аппликатор оставался в пробирке (рис. 4).
После самостоятельного отбора проб влагалищной жидкости (ВЖ) системами «Я сама» и «Qintip» врачом проводился гинекологический осмотр, во время которого для контроля качества самозабора материала пациентками осуществляли классическое взятие биоматериала для диагностики ИППП. С этой целью тампоном убирали слизь и ВЖ с шейки матки и брали контрольный соскоб из заднего свода влагалища (диагностика наличия инфекций), и соскоб из цервикального канала – контроль наличия ВПЧ. Полученный биоматериал помещали в пробирки типа «Эппендорф» с транспортной средой с маркировкой «Ц» для шейки матки и без маркировки – влагалище (рис. 5).
 Все 4 полученных образца в течение суток отправляли в лабораторию «ИНВИТРО», где проводились идентификация микроорганизмов и вирусов.
Все 4 полученных образца в течение суток отправляли в лабораторию «ИНВИТРО», где проводились идентификация микроорганизмов и вирусов.
Расчет положительных образцов производили с учетом специфического положительного сигнала при цикле амплификации, считающимся положительным до 40 цикла для хламидий, M. genitalium, трихомонад, ВПЧ 16-го и 18-го типов, а также до 35-го цикла для всех остальных типов ВПЧ.
В процессе обработки статистических данных применены методы описательной статистики, расчеты проводили на базе прикладных программ Microsoft Excel и Statistica 12.0. Отношение шансов (ОШ) и значимость различий в частоте встречаемости качественных признаков проводили по критерию χ2. ОШ приведено с 95% доверительным интервалом (ДИ).
Результаты исследования
Одним из показателей достоверности получаемых результатов является анализ качества взятия материала (КВМ), который представляет собой тест по определению количества геномной ДНК человека в биоматериале, источником которой являются эпителиальные клетки, попадающие в пробу при правильной технике взятия биоматериала. Определяется в копиях ДНК человека в пробе. Качество взятия считается удовлетворительным, если КВМ ≥4 lg (10 000 копий в образце). Для выявления ВПЧ это принципиальное условие получения достоверного результата. В табл. 1 представлены результаты КВМ по всем использованным методикам.
Полученные результаты свидетельствуют, что показатель КВМ по исследованным методикам сопоставим по уровням ≥4 lg (р>0,005), то есть не имел статистически значимых различий.
Из 110 пациенток Ch. trachomatis выявлены у 2 женщин (табл. 2) в вагинальном и цервикальном мазках, взятых врачом. Также у этих же пациенток методом самозабора «Qvintip» и в 1 наблюдении методом «Я сама». Статистически достоверной разницы не обнаружено. Другие возбудители ИППП – гонококки, трихомонады, M. genitalium не были выявлены ни у одной из обследованных женщин с использованием изучаемых и контролируемых методик. Таким образом, частота выявления инфекций сопоставима как при взятии мазка врачом из влагалища и цервикального канала, так и с использованием тест систем «Я сама» и «Qvintip» (р>0,005).
Вирусы папилломы человека различных видов используемыми методами были выявлены у 23 из 110 обследованных женщин, что составило 20,9%. В табл. 3 представлены результаты их обнаружения.

Всего из 22 тестируемых типов ВПЧ было выявлено 11. По одному типу ВПЧ обнаружено у 15 женщин, сочетание двух типов – у 6 и трех типов – у двух пациенток. Таким образом, было идентифицировано 33 вируса папилломы человека.
Полное совпадение изучаемых и сравниваемых методов отмечено у 108 пациенток. Несовпадение выделения вирусов констатировано лишь в двух наблюдениях по ВПЧ 33 и 68 типам. У одной женщины ВПЧ 33 выделен однократно из цервикального канала при отборе материала врачом, но ни в одном из других образцов нативного материала вирус не выделен. У другой пациентки ВПЧ 68 выявлен из цервикального канала и влагалища при взятии врачом, но при обоих методах самозабора нет.
В целом же, частота выявления инфекций сопоставима (р>0,05) при взятии мазка из влагалища тест-системами «Я сама» и «Qvintip». Расхождение отмечено всего в двух случаях, что статистически недостоверно и может интерпретироваться как качество взятия материала, сопоставимо по уровню ≥4,00 (р>0,05). Следовательно, использование систем «Qvintip» и «Я сама» может применяться для диагностики инфекций.
Полученные результаты убедительно показали, что самостоятельный забор биоматериала из влагалища непосредственно пациентками по своей диагностической значимости не уступает методам забора материала, осуществляемого врачом во время гинекологического осмотра. Более того, отечественная тест-система «Я сама» (Россия) оказалась по всем параметрам качества сопоставима с зарубежной аналогичной системой «Qvintip» (Швеция), но по программе импортозамещения экономически более привлекательна. Расхождение результатов отмечено лишь в единичных случаях по некоторым видам ВПЧ, что было статистически незначимо. Важно подчеркнуть, что самостоятельный отбор проб нативного материала влагалища сокращает сроки скрининга населения на инфекции, ассоциированные с ВПЧ, позволяет охватить большую часть женского населения и тем самым улучшить возможности ранней диагностики и профилактики ВПЧ-ассоциированных заболеваний, включая рак шейки матки.

Обсуждение
Выполненное исследование показало, что более чем каждая пятая женщина (20,9%) репродуктивного возраста без каких-либо жалоб инфицирована ВПЧ, в том числе и онкогенными типами. Поэтому актуальной задачей современного здравоохранения является раннее выявление ассоциированных с ВПЧ заболеваний и, в первую очередь рака шейки матки, вульвы и влагалища. В связи с этим совершенствование ранней диагностики ВПЧ-ассоциированных заболеваний относится к чрезвычайно важному направлению развития современной гинекологии. Некоторые зарубежные исследования свидетельствуют, что многие женщины предпочитают не участвовать в регулярном скрининге рака шейки матки. 65% всех случаев рака шейки матки находят у этих женщин [4]. Около 30% женщин, приглашенных на скрининг, не приходят на обследование. Эти данные идентичны во всех странах, в которых есть программы скрининга в течение последних 30–50 лет. M. Oscarsson и соавт. (2007) выделяют несколько наиболее распространенных причин, по которым женщины не участвуют в скрининге: 1. Недостаток времени в связи с семьей или работой; 2. Чувство дискомфорта, стресса при гинекологическом осмотре; 3. Страх обнаружения рака; 4. Чувствуют себя здоровыми [5].
Для того, чтобы преодолеть низкую посещаемость скрининга на ВПЧ-инфекции и рак шейки матки, в частности в Швеции, был разработан специальный комплект («Qvintip») для самостоятельного забора пробы влагалищного содержимого дома с последующей отправкой пробирки по почте в лабораторию, где уже и проводилось типирование биоматериала на ВПЧ. В Упсальском университете было проанализировано >10 000 проб самозабора ВЖ. Было установлено, что у женщин моложе 30 лет, частота инфекций ВПЧ была выше, чем ASCUS (при выявлении по мазку ПАП). Однако у женщин старше 40 лет ВПЧ тест является более чувствительным, чем цитологическое исследование [6]. Разработанная система с фирменным программным обеспечением для отслеживания всего процесса и результаты этих исследований стали основой для государственной программы Швеции по раннему скринингу ВПЧ-ассоциированных заболеваний с регулярными отчетами и информацией о ВПЧ-положительных и ВПЧ-отрицательных женщинах.
В нашей стране похожее исследование было выполнено Н.В. Артымук и К.В. Марочко (2016), в котором изучалась возможность самостоятельного отбора проб ВЖ для идентификации ВПЧ. Полученные авторами результаты свидетельствовали не только о высокой частоте инфицированности ВПЧ высокого онкогенного риска среди заключенных женщин, но и о высокой прогностической ценности метода самостоятельного отбора биоматериала с помощью системы «Qvintip» [7].
Заключение
Полученные данные свидетельствуют о высокой частоте инфицирования ВПЧ молодых здоровых женщин (20,9%) несколькими генотипами вирусов, среди которых часто встречаются и онкогенные типы. Сравнительный анализ двух методов самозабора биоматериала из влагалища показал их идентичность врачебным манипуляциям и высокую достоверность результатов исследования. В отличие от зарубежного аналога, отечественная тест-система «Я сама» имела несомненные преимущества, в том числе и экономического характера. Указанное устройство может быть рекомендовано в реализации программ скрининга ВПЧ-ассоциированных заболеваний в широкой клинической практике.



