Проблема недостаточного ответа яичников на стимуляцию гонадотропинами в программах экстракорпорального оплодотворения (ЭКО) у женщин репродуктивного возраста со сниженными показателями овариального резерва волнует специалистов на протяжении длительного времени [1]. Женщины со сниженными показателями овариального резерва составляют 24–48% всех пациенток, подвергающихся стимуляции функции яичников в программах вспомогательных репродуктивных технологий [2, 3]. В анамнезе у данной группы пациенток отмечаются неоднократные неэффективные попытки лечения в программах ЭКО/интрацитоплазматической инъекции сперматозоида (ИКСИ), что оказывает влияние как на их психологическое состояние, так и на социальное положение в обществе.
«Волновая теория» развития фолликулов в яичниках явилась основой для проведения стимуляции суперовуляции не только в фолликулярной фазе, но и в лютеиновой фазе цикла у женщин со сниженным овариальным резервом [4].
В 2014 г. Y. Kuang с соавт. ввели понятие «двойная стимуляция», подразумевающее стимуляцию в фолликулярную и в лютеиновую фазу в рамках одного менструального цикла [5]. Результаты исследований ряда авторов показали, что двойная стимуляция является эффективным подходом для лечения бесплодия у пациентов со снижением овариального резерва [2, 5].
В настоящее время остается актуальным вопрос о качестве ооцитов и эмбрионов, получаемых в результате стимуляции функции яичников в различные фазы менструального цикла. Известно, что в современной практике качество переносимых эмбрионов в основном оценивается согласно морфологическим критериям, таким как скорость, своевременность и равномерность деления клеток, форма бластомеров, степень их фрагментации и другие [6]. Однако точность такого метода отбора эмбрионов недостаточно высокая, и в последние годы появляется все больше исследований, демонстрирующих недостатки морфологического критерия оценки качества эмбрионов [7, 8]. В связи с этим возникает необходимость поиска новых методов, с помощью которых возможно определение качества эмбрионов и их потенциала к имплантации.
Данные проведенных на сегодняшний день исследований свидетельствуют о том, что кумулюсные клетки могут быть использованы для определения потенциала развития ооцитов, оценки качества эмбриона и эффективности протокола стимуляции яичников [8–11].
Известно, что кумулюсные клетки, окружающие и питающие ооцит, образуются в процессе фолликулогенеза на стадии формирования полости внутри фолликула из низкодифференцированных предшественников – клеток гранулезы [12, 13].
Кумулюсные клетки тесно связаны с ооцитом посредством специальных щелевых контактов, позволяющих осуществлять метаболический обмен и транспорт сигнальных молекул [14, 15]. Таким образом, кумулюсные клетки: 1) координируют созревание ооцитов с развитием фолликулов; 2) оказывают влияние на ядерное и цитоплазматическое созревание ооцита; 3) обеспечивают энергией процесс возобновления мейотического созревания ооцита; 4) стимулируют гликолиз, аминокислотный транспорт и биосинтез стеролов [13, 16].
По результатам проведенных на сегодняшний день исследований доказано, что уровень экспрессии потенциально значимых генов в клетках кумулюса коррелирует с показателями качества ооцитов, развитием эмбрионов на предимплантационном этапе и частотой наступления беременности [8, 17–21]. Однако известно, что данные исследования проводились исключительно в группах женщин с нормальным овариальным резервом.
Таким образом, нами впервые была проведена оценка транскрипционного профиля в клетках кумулюса у пациенток со сниженным овариальным резервом.
Для проведения исследования мы выбрали гены, участвующие в контроле процессов клеточной репродукции (CALM2, ITPKA, SPSB2), передачи внутриклеточной информации и формировании внеклеточного матрикса (SDC4, HAS2, PTGS2(COX2), GREM1), миграции, пролиферации клеток и ангиогенеза (ALCAM (CD 166), VCAN), программируемой гибели клеток (TP53I3), следовательно, потенциально связанные с процессами фолликулогенеза, оогенеза и развитием эмбрионов.
Цель исследования: изучить возможность использования транскрипционного профиля в клетках кумулюса для прогноза качества эмбрионов у женщин со сниженным овариальным резервом в протоколах стимуляции суперовуляции в фолликулярную и лютеиновую фазы менструального цикла.
Материал и методы исследования
На базе отделения сохранения и восстановления репродуктивной функции и лаборатории молекулярно-генетических методов ФГБУ Научный центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России проведено проспективное исследование случай-контроль.
В кумулюсных клетках методом полимеразной цепной реакции с обратной транскрипцией (ОТ-ПЦР) в режиме реального времени был исследован уровень экспрессии мРНК 10 генов: генов внеклеточного матрикса (гиалуронан-синтетаза 2 (HAS2), версикан (VCAN), молекула клеточной адгезии активированных лейкоцитов (ALCAM или CD166)), генов, регулирующих передачу внутриклеточной информации (простагландин синтетаза 2 (PTGS2), гремлин (GREM1), инозитол-трифосфат 3 киназы А (ITPKA), синдекан 4 (SDC4), кальмодулина (CALM2)), а также гена, регулирующего убиквитинирование (супрессор цитокиновой сигнализации, содержащий SPRY-домен (SPSB2)) и гена опухолевого протеина (TP53I3) (реактивы ДНК-Технология, Россия).
Во избежание деградации РНК взятие материала (кумулюсные клетки) осуществляли в пробирки с раствором гуанидинтиоцианата (лизирующий раствор наборы «Проба НК»). Осаждение РНК проводили изопропанолом в присутствии соосадителя. В реакции обратной транскрипции использовали смесь специфических олигонуклеотидов всех исследуемых генов. Амплификацию осуществляли в режиме реального времени с измерением уровня флуоресценции по каналу FAM на каждом цикле при температуре 64°С. Реализация «горячего старта» обеспечивалась использованием Taq-полимеразы, активность которой блокировалась антителами и восстанавливалась при прогреве. Реакцию ставили в двух повторах для каждой точки. Уровень экспрессии измеряли в условных единицах относительно референсных генов TBP, B2M, GUSB методом сравнения пороговых циклов (∆Cq).
 В исследование были включены 160 образцов кумулюсных клеток, полученных от 40 пациенток, проходивших лечение в программе ЭКО/ИКСИ и соответствовавших критериям включения (возраст 25–42 лет; уровень антимюллерова гормона (АМГ) <1,2 нг/мл, базальная концентрация фолликулостимулирующего гормона (ФСГ) >11 мМЕ/мл, <20 мМЕ/мл; наличие в анамнезе безуспешных циклов ЭКО/ИКСИ; индекс массы тела (ИМТ) от 18 до 29 кг/м2 (включительно)). Пациентки были разделены на две группы с учетом фазы, во время которой проводилась программа ЭКО: I группа (n=17) – стимуляция суперовуляции проводилась в фолликулярной фазе, II группа (n=23) – проводилась стимуляция препаратами гонадотропинов в лютеиновой фазе менструального цикла. Оплодотворение проводилось методом ИКСИ. Оценку качества эмбрионов производили на 3-и сутки (через 72 часа после получения ооцитов) после оплодотворения на основании совокупности их морфологических характеристик. При этом учитывались показатели скорости дробления эмбрионов, оценивались симметричность бластомеров, их моно-/мультинуклеарность и степень фрагментации. На 3-и сутки эмбрионы, не имевшие никаких морфологических аномалий, получали максимальную оценку в 3,5 балла. Каждое отклонение от нормальных параметров в морфологии эмбриона снижало оценку на 0,5 балла. При переносе на 3-и сутки выбирались 8-клеточные эмбрионы с оценкой не менее 2,5 балла (эмбрионы отличного и хорошего качества).
В исследование были включены 160 образцов кумулюсных клеток, полученных от 40 пациенток, проходивших лечение в программе ЭКО/ИКСИ и соответствовавших критериям включения (возраст 25–42 лет; уровень антимюллерова гормона (АМГ) <1,2 нг/мл, базальная концентрация фолликулостимулирующего гормона (ФСГ) >11 мМЕ/мл, <20 мМЕ/мл; наличие в анамнезе безуспешных циклов ЭКО/ИКСИ; индекс массы тела (ИМТ) от 18 до 29 кг/м2 (включительно)). Пациентки были разделены на две группы с учетом фазы, во время которой проводилась программа ЭКО: I группа (n=17) – стимуляция суперовуляции проводилась в фолликулярной фазе, II группа (n=23) – проводилась стимуляция препаратами гонадотропинов в лютеиновой фазе менструального цикла. Оплодотворение проводилось методом ИКСИ. Оценку качества эмбрионов производили на 3-и сутки (через 72 часа после получения ооцитов) после оплодотворения на основании совокупности их морфологических характеристик. При этом учитывались показатели скорости дробления эмбрионов, оценивались симметричность бластомеров, их моно-/мультинуклеарность и степень фрагментации. На 3-и сутки эмбрионы, не имевшие никаких морфологических аномалий, получали максимальную оценку в 3,5 балла. Каждое отклонение от нормальных параметров в морфологии эмбриона снижало оценку на 0,5 балла. При переносе на 3-и сутки выбирались 8-клеточные эмбрионы с оценкой не менее 2,5 балла (эмбрионы отличного и хорошего качества).
Морфологическая оценка качества эмбрионов на 5-е сутки была произведена согласно классификации D. Gardner и W.B. Schoolcraft (1999) [22]. Стадии развития бластоцисты:
- 0 – Не бластоциста.
- 1 – Ранняя бластоциста: бластоцель составляет менее 50% от объема всей бластоцисты.
- 2 – Ранняя бластоциста: бластоцель составляет 50–80% объема всей бластоцисты.
- 3 – Полностью развитая бластоциста, большая бластоцель.
- 4 – Экспандированная бластоциста – более чем полностью развитая бластоциста, тонкая zona pellucida (ZP).
- 5 – Бластоциста, начавшая хетчинг, то есть трофэктодерма бластоцисты начинает прорывать ZP.
- 6 – Бластоциста, закончившая хетчинг, то есть полностью вышедшая из ZP.
Внутриклеточная масса (ВКМ):
- A – Плотно упакованное большое количество клеток.
- B – Свободно сгруппированное небольшое количество клеток.
- C – Очень мало клеток или практически не определяется.
Трофэктодерма (ТФЭ):
- A – Много клеток, регулярное строение.
- B – Немного клеток, нерегулярное строение.
- C – Очень мало клеток и поврежденные клетки.
Все кумулюсы ооцитов были разделены на 2 группы в зависимости от фазы проведенной стимуляции функции яичников. I группа – кумулюсы ооцитов, полученные в протоколе лечения в фолликулярной фазе (70 образцов), II группа – кумулюсы ооцитов, полученные в протоколе лечения в лютеиновой фазе двойной стимуляции (90 образцов), из исследования выбыло 4 образца второй группы в связи с неудовлетворительным качеством РНК, 16 образцов кумулюса незрелых ооцитов (7 в первой группе и 9 образцов во второй). С целью выявления взаимосвязи уровня экспрессии мРНК генов и показателей качества эмбрионов у женщин со сниженным овариальным резервом в программах ЭКО/ИКСИ полученные кумулюсные клетки были разделены на три класса в зависимости от качества эмбрионов согласно морфологическим критериям оценки: 1-й класс – эмбрионы хорошего качества (n=42), 2-й класс – эмбрионы удовлетворительного качества (n=43), 3-й класс – эмбрионы неудовлетворительного качества (n=17). Из данного этапа исследования были исключены 38 образцов, из которых 23 – неоплодотворившиеся ооциты и 15 – остановившиеся в развитии эмбрионы. Исследование было одобрено комиссией по этике биомедицинских исследований ФГБУ НЦАГиП им. В.И. Кулакова МЗ РФ. Статистическая обработка данных выполнена при помощи пакета прикладных программ SPSS Statistics 22.0.
Для оценки характера распределения количественных данных предварительно проводился тест Колмогорова–Смирнова. Поскольку в большинстве случаев распределение данных было отлично от нормального, использовались методы непараметрической статистики. Для определения достоверности различий использован критерий Манна–Уитни для несвязанных совокупностей.
Статистическую обработку данных производили общепринятыми методами вариационной статистики. Для каждого количественного параметра были определены среднеарифметическое значение, стандартное среднеквадратичное отклонение и стандартная ошибка среднего, медиана (Me), верхний (H) и нижний квартили (L).
Достоверность различий в частоте встречаемости качественных признаков определяли по критерию χ2. Статистически значимыми считались различия при р<0,05.
Модель прогноза вероятности получения эмбриона хорошего качества получена при помощи метода логистической регрессии. Уравнение для предсказания вероятности наступления анализируемого события (получения эмбрионов отличного качества) имела вид:
Р = 1/(1+e-z) *100% (формула 1),
где
z – значение классифицирующей функции, рассчитанное по формуле 2.
z = a + b1*X1 + b2*Х2+ ...+ bn*Xn, (формула 2),
где:
а – константа;
Xi – независимые переменные (уровень экспрессии генов); bi – коэффициенты.
При значениях p (искомой вероятности наступления события) менее 0,5 предполагалось, что измеряемое событие не наступит; в противном случае предполагалось наступление события. Для валидации полученной модели и выбора точки cut-off с требованием наибольшей суммарной чувствительности и специфичности метода проводился ROC-анализ.
Результаты исследования
Средний возраст пациенток в I группе составил 35,32±4,3 года, во II группе – 35,11±3,2 года; ИМТ в I группе – 21,4±2,2 кг/м2, во II группе – 21,2±3,1 кг/м2 соответственно (р>0,05) (табл. 1).

Также не выявлено статистически достоверных различий в продолжительности стимуляции суперовуляции и суммарной дозе гонадотропина (р>0,05). Среднее число ооцитов в обеих группах статистически достоверно не различалось (р>0,05). Количество бластоцист составило 1,6±1,4 и 1,9±1,8 соответственно (р>0,05).
В ходе проведенного анализа образцов было выявлено статистически значимое повышение уровня экспрессии мРНК генов VCAN, SDC4 и TP53I3 в клетках кумулюса во II группе исследования (р=0,003, p=0,005, p<0,001 соответственно) (табл. 2).
Далее был проведен анализ уровней экспрессии мРНК исследуемых генов в кумулюсных клетках в зависимости от качества полученных эмбрионов у женщин со сниженным овариальным резервом в программе ЭКО/ИКСИ (табл. 3).
Так, в кумулюсных клетках эмбрионов хорошего качества было выявлено повышение уровня экспрессии мРНК генов HAS2 в 1,8 раза, VCAN в 2 раза и PTGS2 в 2,9 раза, и снижение уровня мРНК гена ITPKA в 1,9 раза (р<0,05).
Для выявления молекулярно-генетических маркеров, ассоциированных с качеством эмбрионов, согласно морфологическим критериям оценки на 3-и и 5-е сутки культивирования методом логистической регрессии была построена модель, исходом в которой была переменная, характеризующая получение эмбрионов хорошего качества, предикторами – уровни экспрессии мРНК генов HAS2, PTGS2, VCAN.
Уравнение дискриминирующей (классифицирующей) функции имело вид:
z = 1,786*ln[HAS2]+0,925*ln[PTGS2]+1,55*ln[VCAN] – 1,351 (формула 3),
где [HAS2], [PTGS2], [VCAN] – нормированные уровни экспрессии соответствующих генов относительно референсных генов B2M, GUSB, TBP, рассчитанные с помощью метода сравнения пороговых циклов (∆Ср).
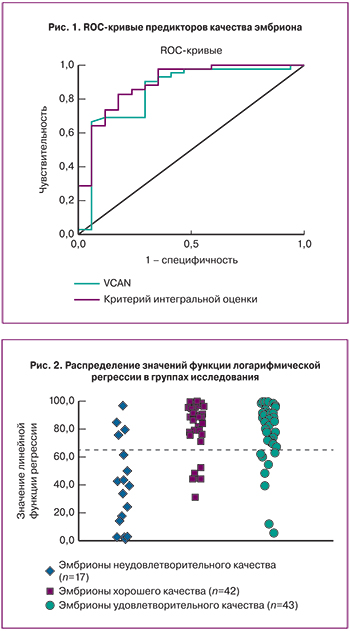
Наибольший вклад в уравнение дискриминирующей функции внес ген VCAN. Данный маркер может быть использован в качестве независимого маркера для оценки качества эмбрионов у женщин со сниженным овариальным резервом. При уровне отсечки положительного результата 2,46 о.е. чувствительность и специфичность метода соответственно составили 90,5 и 70,6%, значение площади под ROC-кривой AUC=0,845±0,064 (p=3,7*10-5), что незначительно уступало критерию интегральной оценки (рис. 1).
Распределение образцов по группам в соответствии с предложенной моделью представлено на рис. 2 и в табл. 4. Большая часть эмбрионов хорошего качества классифицировались как отличные (81,4%).
При анализе частоты наступления клинической беременности при переносе эмбрионов хорошего качества статистических различий между исследуемыми группами выявлено не было (p=0,08). В группе стимуляции суперовуляции в фолликулярной фазе беременность наступила в 23,8% (n=5), в группе стимуляции в лютеиновой фазе – в 33,3% (n=7) случаев переноса эмбрионов.
Обсуждение
Лечение бесплодия у женщин со сниженным овариальным резервом путем проведения стимуляции функции яичников в различные фазы менструального цикла на данный момент является предметом изучения. На сегодняшний день в мире еще не представлены данные о качестве ооцитов и эмбрионов, полученных в результате проведения подобных протоколов стимуляции суперовуляции.
В поисках новых малоинвазивных методик оценки качества ооцитов и эмбрионов в программе ВРТ ученые остановили свое внимание на оценке уровня транскрипционной активности в кумулюсных клетках. В нашем исследовании мы провели анализ уровня экспрессии наиболее значимых в клеточной репродукции генов.
Так, в проведенном нами исследовании при сравнении между группами было выявлено статистически значимое повышение уровня экспрессии мРНК генов VCAN, SDC4, и TP53I3 в клетках кумулюса в группе проведения программы ЭКО в лютеиновой фазе (р=0,003, p=0,005, p<0,001 соответственно). Известно, что ген версикан (VCAN) относится к семейству протеогликанов и является одним из основных компонентов внеклеточного матрикса [23].

Этот белок участвует в процессе клеточной адгезии, а также принимает участие в пролиферации, дифференцировке и миграции клеток, ангиогенезе, апоптозе и играет чрезвычайно важную роль в формировании структуры тканей и стабилизации белков внеклеточного матрикса [24–26]. Также согласно построенной логистической регрессионной модели, в которой исходом была переменная, характеризующая получение эмбрионов хорошего качества, а предикторами – уровни экспрессии мРНК исследуемых генов, уровень экспрессии мРНК гена VCAN является наиболее информативным маркером для оценки качества эмбрионов согласно морфологическим критериям оценки в программе ЭКО. Проведенный ROC-анализ продемонстрировал достаточно высокую точность построенной модели с площадью под кривой 0,845, чувствительность и специфичность метода составили 90,5 и 70,6% соответственно. Эти данные подтверждают исследования Wathlet и соавт., где уровень экспрессии гена VCAN был значительно выше в кумулюсных клетках ооцитов, впоследствии при оплодотворении которых развились эмбрионы хорошего качества (p<0,05) [20].
Ген синдекан 4 (риудокан) (SDC4) кодирует белок, являющийся трансмембранным гепарансульфатным протеогликаном, который функционирует в качестве рецептора для передачи внутриклеточной информации. Показано, что SDC4 играет немаловажную роль в воспалительной реакции и ангиогенезе [27]. Он также ингибирует апоптоз клеток, опосредованный фактором некроза опухоли [28]. По данным литературы также известно, что синдекан 4 может выполнять несколько биологических функций, включая пролиферацию клеток и повышение антикоагуляционной активности; он также вовлечен в процесс созревания фолликулов и участвует в процессе овуляции [29].
По данным литературы известно, что ген TP53I3 (PIG3) кодирует подсемейство белков, схожих с оксиредуктазами, которые участвуют в клеточных реакциях в ответ окислительный стресс [30]. Этот ген стимулируется супрессором опухоли р53, принимает непосредственное участие в ответе на повреждение ДНК и, как полагают, участвует в p53-опосредованной гибели клеток [31, 32]. Таким образом, снижение уровня экспрессии мРНК гена TP53I3 может приводить к снижению реакции на повреждение ДНК, тем самым вызывая дезорганизацию течения различных процессов, в том числе апоптоза. Последнее, в свою очередь, приводит к сохранению кумулюсных клеток, не способных выполнять свою основную функцию: метаболический обмен и транспорт сигнальных молекул, что приводит к нарушению созревания ооцита [33].
Известно, что ген HAS2 является основным из трех изоформ (HAS1, HAS2, HAS3) ферментом, необходимым для синтеза гиалуроновой кислоты кумулюсными клетками, которая в свою очередь является главным компонентом внеклеточного матрикса, связывающего вместе ооцит и кумулюсные клетки [34–36]. Гиалуроновая кислота имеет важное значение в процессах овуляции, оплодотворения и эмбриогенеза [37–39]. Ген HAS2 стимулирует клеточную пролиферацию, инициирует передачу внутриклеточной информации, а также участвует в процессе ангиогенеза и обладает иммуностимулирующим действием [40–42].
Ген COX2, также известный как простагландин-синтетаза 2 (PTGS2), играет важную роль, принимая участие в процессах овуляции, оплодотворения, имплантации и родах [43–45]. Экспрессия PTGS2 регулируется ооцит-секретируемым паракринным фактором роста и дифференцировки-9 (GDF9 – growth differentiation factor), и наряду с геном HAS2 он участвует в передаче внутриклеточной информации и формировании внеклеточного матрикса [8, 18].
McKenzie и соавт. в своем исследовании показали, что уровень экспрессии мРНК генов HAS2 и PTGS2 был в 6 раз выше в кумулюсных клетках ооцитов, развившихся до эмбрионов хорошего качества, по сравнению с эмбрионами плохого качества (р<0,05) [46]. В ходе нашего исследования при анализе эмбрионов хорошего и плохого качества отмечалось повышение уровня экспрессии мРНК гена HAS2 в 1,8 раза и гена PTGS2 в 2,9 раза в кумулюсных клетках эмбрионов хорошего качества (р=0,001). Похожие данные были также представлены в исследовании Cillo и др., продемонстрировавших повышение уровня экспрессии мРНК генов HAS2 в кумулюсных клетках эмбрионов хорошего качества по сравнению с эмбрионами плохого качества (р<0,05) [47].
Заключение
Таким образом, результаты проведенного исследования позволяют предположить, что стимуляция суперовуляции в лютеиновой фазе менструального цикла не оказывает отрицательного влияния на потенциал развития ооцитов и качество эмбрионов. Также уровни экспрессии мРНК генов VCAN, HAS2 и PTGS2 могут быть использованы как предикторы хорошего качества развивающихся эмбрионов у женщин со сниженным овариальным резервом.
Необходимо дальнейшее исследование факторов, влияющих на качество ооцитов и эмбрионов и, следовательно, на исход программ ВРТ у пациенток со сниженными параметрами овариального резерва при проведении протоколов стимуляции в различные фазы менструального цикла с целью повышения эффективности лечения бесплодия.



